Bioassay-guided isolation and identification of cytotoxic compounds from Gymnosperma glutinosum leaves
- PMID: 22996346
- PMCID: PMC6268992
- DOI: 10.3390/molecules170911229
Bioassay-guided isolation and identification of cytotoxic compounds from Gymnosperma glutinosum leaves
Abstract
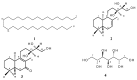
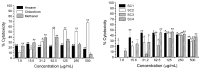
Bioassay-guided fractionation of hexane extracts of Gymnosperma glutinosum (Asteraceae) leaves, collected in North Mexico, afforded the known compounds hentriacontane (1) and (+)-13S,14R,15-trihydroxy-ent-labd-7-ene (2), as well as the new ent-labdane diterpene (-)-13S,14R,15-trihydroxy-7-oxo-ent-labd-8(9)-ene (3). In addition, D-glycero-D-galactoheptitol (4) was isolated from the methanolic extract of this plant. Their structures were established on the basis of high-field 1D- and 2D NMR methods supported by HR-MS data. The cytotoxic activity was determined by using the in vitro L5178Y-R lymphoma murine model. Hentriacontane (1) and the new ent-labdane 3 showed weak cytotoxicity, whereas the ent-labdane 2 showed significant (p < 0.05) and concentration dependent cytotoxicity (up to 78%) against L5178Y-R cells at concentrations ranging from 7.8 to 250 μg/mL.
Figures
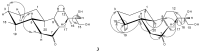

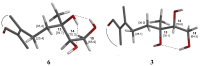

Similar articles
-
Long-chain alkanes and ent-labdane-type diterpenes from Gymnosperma glutinosum with cytotoxic activity against the murine lymphoma L5178Y-R.Phytother Res. 2012 Nov;26(11):1632-6. doi: 10.1002/ptr.4625. Epub 2012 Feb 23. Phytother Res. 2012. PMID: 22359371
-
Antitumor properties of Gymnosperma glutinosum leaf extracts.Cancer Invest. 2009 Feb;27(2):149-55. doi: 10.1080/07357900802192190. Cancer Invest. 2009. PMID: 19235586
-
A cytotoxic diterpene from Alomia myriadenia.Phytochemistry. 2000 Apr;53(8):877-80. doi: 10.1016/s0031-9422(99)00564-6. Phytochemistry. 2000. PMID: 10820796
-
A Comprehensive View on (-)-7-Oxo-ent-kaur-16-en-19-oic Acid, the Major Constituent of Xylopia sericea Leaves Extract: Complete NMR Assignments, X-Ray Crystallographic Structure, in Vitro Antimalarial Activity and Cytotoxicity.Chem Biodivers. 2019 Jul;16(7):e1900141. doi: 10.1002/cbdv.201900141. Epub 2019 Jun 12. Chem Biodivers. 2019. PMID: 31087758
-
ent-trachyloban-3beta-ol, a new cytotoxic diterpene from Croton zambesicus.Planta Med. 2002 Jul;68(7):647-9. doi: 10.1055/s-2002-32903. Planta Med. 2002. PMID: 12143003
Cited by
-
Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis.Molecules. 2014 Dec 15;19(12):21044-65. doi: 10.3390/molecules191221044. Molecules. 2014. PMID: 25517343 Free PMC article.
-
Pharmacological evaluation of 2-angeloyl ent-dihydrotucumanoic acid.Pharm Biol. 2017 Dec;55(1):873-879. doi: 10.1080/13880209.2016.1277766. Pharm Biol. 2017. PMID: 28142303 Free PMC article.
-
Defensive Armor of Potato Tubers: Nonpolar Metabolite Profiling, Antioxidant Assessment, and Solid-State NMR Compositional Analysis of Suberin-Enriched Wound-Healing Tissues.J Agric Food Chem. 2015 Aug 5;63(30):6810-22. doi: 10.1021/acs.jafc.5b03206. Epub 2015 Jul 24. J Agric Food Chem. 2015. PMID: 26166447 Free PMC article.
-
Nanoencapsulation of n-butanol extract of Symphytum kurdicum and Symphytum asperrimum: Focus on phytochemical analysis, anti-oxidant and antibacterial activity.Iran J Basic Med Sci. 2022 Mar;25(3):364-371. doi: 10.22038/IJBMS.2022.62032.13760. Iran J Basic Med Sci. 2022. PMID: 35656178 Free PMC article.
-
Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México.PeerJ. 2018 Feb 9;6:e4358. doi: 10.7717/peerj.4358. eCollection 2018. PeerJ. 2018. PMID: 29441241 Free PMC article.
References
-
- Efferth T. Cancer therapy with natural products and medicinal plants. PlantaMed. 2010;76:1035–1036. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

