Down-modulation of expression, or dephosphorylation, of IG20/MADD in tumor necrosis factor-related apoptosis-inducing ligand-resistant thyroid cancer cells makes them susceptible to treatment with this ligand
- PMID: 22998497
- PMCID: PMC3539253
- DOI: 10.1089/thy.2012.0155
Down-modulation of expression, or dephosphorylation, of IG20/MADD in tumor necrosis factor-related apoptosis-inducing ligand-resistant thyroid cancer cells makes them susceptible to treatment with this ligand
Abstract
Background: The IG20/MADD gene is overexpressed in thyroid cancer tissues and cell lines, and can contribute to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance. The ability of the MADD protein to resist TRAIL-induced apoptosis is dependent upon its phosphorylation by Akt. Interestingly, while TRAIL induces a significant reduction in the levels of phospho-Akt (pAkt) and phospho-MADD (pMADD) in TRAIL-sensitive cells, it fails to do so in TRAIL-resistant cells. In this study, we investigated if MADD phosphorylation by Akt was contributing to TRAIL resistance in thyroid cancer cells.
Methods: We determined the susceptibility of different thyroid cancer cell lines to TRAIL-induced apoptosis by fluorescence-activated cell sorting (FACS) analysis. We tested for various TRAIL resistance factors by FACS analyses or for IG20/MADD expression by quantitative reverse transcription-polymerase chain reaction. We determined the levels of pAkt and pMADD upon TRAIL treatment in thyroid cancer cells by Western blotting. We tested if down-modulation of IG20/MADD gene expression using shRNA or phosphorylation using a dominant negative Akt (DN-Akt) or pretreatment with LY294002, a PI3 kinase inhibitor, could help overcome TRAIL resistance.
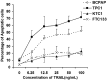
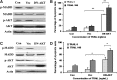
Result: BCPAP and TPC1 cells were susceptible, while KTC1 and FTC133 cells were resistant, to TRAIL-induced apoptosis. The differential susceptibility to TRAIL was not related to the levels of expression of death receptors, decoy receptors, or TRAIL. KTC1 and FTC133 cells showed higher levels of IG20/MADD expression relative to BCPAP and TPC1, and were rendered susceptible to TRAIL treatment upon IG20/MADD knockdown. Interestingly, upon TRAIL treatment, the pAkt and pMADD levels were reduced in TRAIL-sensitive BCPAP and TPC1 cells, while they remained unchanged in the resistant KTC1 and FTC133 cells. While expression of a constitutively active Akt in BCPAP and TPC1 cells rendered them resistant to TRAIL, pretreating KTC1 and FTC133 cells with LY294002 rendered them TRAIL-sensitive. Moreover, expression of a DN-Akt in KTC1 and FTC133 cells reduced the levels of pAkt and pMADD and sensitized them to TRAIL-induced apoptosis.
Conclusion: Our results show that pMADD is an important TRAIL resistance factor in certain thyroid cancer cells and suggest that down-modulation of either IG20/MADD expression or phosphorylation can render TRAIL-resistant thyroid cancer cells sensitive to TRAIL.
Figures




Similar articles
-
MADD silencing enhances anti-tumor activity of TRAIL in anaplastic thyroid cancer.Endocr Relat Cancer. 2019 Jun 1;26(6):551-563. doi: 10.1530/ERC-18-0517. Endocr Relat Cancer. 2019. PMID: 30999276
-
Knockdown of IG20 gene expression renders thyroid cancer cells susceptible to apoptosis.J Clin Endocrinol Metab. 2009 Apr;94(4):1467-71. doi: 10.1210/jc.2008-2378. Epub 2009 Feb 3. J Clin Endocrinol Metab. 2009. PMID: 19190106 Free PMC article.
-
Akt-phosphorylated mitogen-activated kinase-activating death domain protein (MADD) inhibits TRAIL-induced apoptosis by blocking Fas-associated death domain (FADD) association with death receptor 4.J Biol Chem. 2010 Jul 16;285(29):22713-22. doi: 10.1074/jbc.M110.105692. Epub 2010 May 18. J Biol Chem. 2010. PMID: 20484047 Free PMC article.
-
Death receptors as targets in cancer.Br J Pharmacol. 2013 Aug;169(8):1723-44. doi: 10.1111/bph.12238. Br J Pharmacol. 2013. PMID: 23638798 Free PMC article. Review.
-
E-cadherin re-expression: Its potential in combating TRAIL resistance and reversing epithelial-to-mesenchymal transition.Gene. 2024 May 30;909:148293. doi: 10.1016/j.gene.2024.148293. Epub 2024 Feb 17. Gene. 2024. PMID: 38373660 Review.
Cited by
-
Role of autophagy in the resistance to tumour necrosis factor-related apoptosis-inducing ligand-induced apoptosis in papillary and anaplastic thyroid cancer cells.Endocrine. 2014 Mar;45(2):256-62. doi: 10.1007/s12020-013-9997-8. Epub 2013 Jul 3. Endocrine. 2014. PMID: 23821365
-
Therapeutic advances in anaplastic thyroid cancer: a current perspective.Mol Cancer. 2018 Oct 23;17(1):154. doi: 10.1186/s12943-018-0903-0. Mol Cancer. 2018. PMID: 30352606 Free PMC article. Review.
-
Combretastatin A4 Regulates Proliferation, Migration, Invasion, and Apoptosis of Thyroid Cancer Cells via PI3K/Akt Signaling Pathway.Med Sci Monit. 2016 Dec 14;22:4911-4917. doi: 10.12659/msm.898545. Med Sci Monit. 2016. PMID: 27966519 Free PMC article.
-
MADD is a downstream target of PTEN in triggering apoptosis.J Cell Biochem. 2014 Feb;115(2):261-70. doi: 10.1002/jcb.24657. J Cell Biochem. 2014. PMID: 24038283 Free PMC article.
-
IG20/MADD plays a critical role in glucose-induced insulin secretion.Diabetes. 2014 May;63(5):1612-23. doi: 10.2337/db13-0707. Epub 2013 Dec 30. Diabetes. 2014. PMID: 24379354 Free PMC article.
References
-
- Schlumberger M. Sherman SI. Clinical trials for progressive differentiated thyroid cancer: patient selection, study design, and recent advances. Thyroid. 2009;19:1393–1400. - PubMed
-
- Nagane M. Huang HJ. Cavenee WK. The potential of TRAIL for cancer chemotherapy. Apoptosis. 2001;6:191–197. - PubMed
-
- Bodmer JL. Holler N. Reynard S. Vinciguerra P. Schneider P. Juo P. Blenis J. Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–243. - PubMed
-
- Wang SH. Mezosi E. Wolf JM. Cao Z. Utsugi S. Gauger PG. Doherty GM. Baker JR., Jr IFNgamma sensitization to TRAIL-induced apoptosis in human thyroid carcinoma cells by upregulating Bak expression. Oncogene. 2004;23:928–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

