Hypoxic enhancement of exosome release by breast cancer cells
- PMID: 22998595
- PMCID: PMC3488584
- DOI: 10.1186/1471-2407-12-421
Hypoxic enhancement of exosome release by breast cancer cells
Abstract
Background: Exosomes are nanovesicles secreted by tumour cells which have roles in paracrine signalling during tumour progression, including tumour-stromal interactions, activation of proliferative pathways and bestowing immunosuppression. Hypoxia is an important feature of solid tumours which promotes tumour progression, angiogenesis and metastasis, potentially through exosome-mediated signalling.
Methods: Breast cancer cell lines were cultured under either moderate (1% O2) or severe (0.1% O2) hypoxia. Exosomes were isolated from conditioned media and quantitated by nanoparticle tracking analysis (NTA) and immunoblotting for the exosomal protein CD63 in order to assess the impact of hypoxia on exosome release. Hypoxic exosome fractions were assayed for miR-210 by real-time reverse transcription polymerase chain reaction and normalised to exogenous and endogenous control genes. Statistical significance was determined using the Student T test with a P value of < 0.05 considered significant.
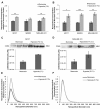
Results: Exposure of three different breast cancer cell lines to moderate (1% O2) and severe (0.1% O2) hypoxia resulted in significant increases in the number of exosomes present in the conditioned media as determined by NTA and CD63 immunoblotting. Activation of hypoxic signalling by dimethyloxalylglycine, a hypoxia-inducible factor (HIF) hydroxylase inhibitor, resulted in significant increase in exosome release. Transfection of cells with HIF-1α siRNA prior to hypoxic exposure prevented the enhancement of exosome release by hypoxia. The hypoxically regulated miR-210 was identified to be present at elevated levels in hypoxic exosome fractions.
Conclusions: These data provide evidence that hypoxia promotes the release of exosomes by breast cancer cells, and that this hypoxic response may be mediated by HIF-1α. Given an emerging role for tumour cell-derived exosomes in tumour progression, this has significant implications for understanding the hypoxic tumour phenotype, whereby hypoxic cancer cells may release more exosomes into their microenvironment to promote their own survival and invasion.
Figures




Similar articles
-
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells.Cell Oncol (Dordr). 2017 Oct;40(5):457-470. doi: 10.1007/s13402-017-0335-7. Epub 2017 Jul 24. Cell Oncol (Dordr). 2017. PMID: 28741069
-
Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1.Oncogene. 2017 Aug 24;36(34):4929-4942. doi: 10.1038/onc.2017.105. Epub 2017 Apr 24. Oncogene. 2017. PMID: 28436951
-
Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT.Mol Cancer. 2019 Mar 13;18(1):40. doi: 10.1186/s12943-019-0959-5. Mol Cancer. 2019. PMID: 30866952 Free PMC article.
-
Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities.Cancer Lett. 2020 Jun 1;479:23-30. doi: 10.1016/j.canlet.2020.03.017. Epub 2020 Mar 19. Cancer Lett. 2020. PMID: 32201202 Review.
-
Hypoxia-Driven Changes in Tumor Microenvironment: Insights into Exosome-Mediated Cell Interactions.Int J Nanomedicine. 2024 Aug 12;19:8211-8236. doi: 10.2147/IJN.S479533. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39157736 Free PMC article. Review.
Cited by
-
Enhancing the Cellular Production of Extracellular Vesicles for Developing Therapeutic Applications.Pharm Res. 2023 Apr;40(4):833-853. doi: 10.1007/s11095-022-03420-w. Epub 2022 Nov 1. Pharm Res. 2023. PMID: 36319886 Free PMC article. Review.
-
Putative Links Between Sleep Apnea and Cancer: From Hypotheses to Evolving Evidence.Chest. 2015 Nov;148(5):1140-1147. doi: 10.1378/chest.15-0634. Chest. 2015. PMID: 26020135 Free PMC article. Review.
-
Extracellular vesicles from human airway basal cells respond to cigarette smoke extract and affect vascular endothelial cells.Sci Rep. 2021 Mar 17;11(1):6104. doi: 10.1038/s41598-021-85534-6. Sci Rep. 2021. PMID: 33731767 Free PMC article.
-
Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis.Front Oncol. 2022 Aug 31;12:966981. doi: 10.3389/fonc.2022.966981. eCollection 2022. Front Oncol. 2022. PMID: 36119470 Free PMC article. Review.
-
Exosome secretion from hypoxic cancer cells reshapes the tumor microenvironment and mediates drug resistance.Cancer Drug Resist. 2022 Jun 21;5(3):577-594. doi: 10.20517/cdr.2022.38. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36176760 Free PMC article. Review.
References
-
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. - PubMed
-
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

