Advances in the development of universal influenza vaccines
- PMID: 22998622
- PMCID: PMC5781207
- DOI: 10.1111/irv.12013
Advances in the development of universal influenza vaccines
Abstract
Despite the widespread availability and use of influenza vaccines, influenza still poses a considerable threat to public health. Vaccines against seasonal influenza do not offer protection against pandemic viruses, and vaccine efficacy against seasonal viruses is reduced in seasons when the vaccine composition is not a good match for the predominant circulating viruses. Vaccine efficacy is also reduced in older adults, who are one of the main target groups for vaccination. The continual threat of pandemic influenza, with the known potential for rapid spread around the world and high mortality rates, has prompted researchers to develop a number of novel approaches to providing immunity to this virus, focusing on target antigens which are highly conserved between different influenza A virus subtypes. Several of these have now been taken into clinical development, and this review discusses the progress that has been made, as well as considering the requirements for licensing these new vaccines and how they might be used in the future.
Keywords: Clinical; influenza; vaccine.
© 2012 John Wiley & Sons Ltd.
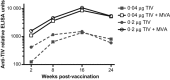
Figures
References
-
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine‐induced antibody response to this strain in the elderly. J Med Virol 2000; 61:94–99. - PubMed
-
- Bodewes R, Rimmelzwaan GF, Osterhaus AD. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines 2010; 9:59–72. - PubMed
-
- Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol 2000; 74:29–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

