Immunoglobulin G (IgG) attenuates neuroinflammation and improves neurobehavioral recovery after cervical spinal cord injury
- PMID: 22998664
- PMCID: PMC3503837
- DOI: 10.1186/1742-2094-9-224
Immunoglobulin G (IgG) attenuates neuroinflammation and improves neurobehavioral recovery after cervical spinal cord injury
Abstract
Background: Evidence suggests that the inflammatory events in the acute phase of spinal cord injury (SCI) exacerbate the initial trauma to the cord leading to poor functional recovery. As a result, minimizing the detrimental aspects of the inflammatory response after SCI is a promising treatment strategy. In this regard, immunoglobulin G (IgG) from pooled human serum is a promising treatment candidate. Due to its putative, though poorly characterized immuno-modulatory effects, IgG has been used clinically to treat neuroinflammatory disorders such as Guillain-Barré syndrome, but its effects in neurotrauma remain largely unexplored.
Methods: This study examines the potential neuroprotective effects of IgG in a well-characterized cervical model of SCI. Female Wistar rats were subject to moderate-severe clip compression injury at the C7-T1 level. IgG (0.4 g/kg) or saline was injected intravenously to randomly selected animals at 15 min post SCI. At several time points post SCI, biochemical assays, histology and immunohistochemistry analyses, and neurobehavioral assessments were used to examine the neuroprotective effects of IgG at the molecular, cellular, and neurobehavioral levels.
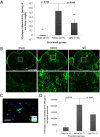
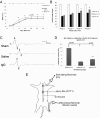
Results: We found that intravenous treatment of IgG following acute clip-compression SCI at C7-T1 significantly reduced two important inflammatory cytokines: interleukin (IL)-1β and IL-6. This early reduction in pro-inflammatory signaling was associated with significant reductions in neutrophils in the spinal cord and reductions in the expression of myeloperoxidase and matrix metalloproteinase-9 in the injured spinal cord at 24 h after SCI. These beneficial effects of IgG were associated with enhanced tissue preservation, improved neurobehavioral recovery as measured by the BBB and inclined plane tests, and enhanced electrophysiological evidence of central axonal conduction as determined by motor-evoked potentials.
Conclusion: The findings from this study indicate that IgG is a novel immuno-modulatory therapy which shows promise as a potential treatment for SCI.
Figures

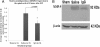


References
-
- Kwon BK, Sekhon LH, Fehlings MG. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine. 2010;Suppl. 1:S263–S270. - PubMed
-
- Blight AR. Delayed demyelination, macrophage invasion: a candidate for “secondary” cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. - PubMed
-
- Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11:13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

