Reduced elastogenesis: a clue to the arteriosclerosis and emphysematous changes in Schimke immuno-osseous dysplasia?
- PMID: 22998683
- PMCID: PMC3568709
- DOI: 10.1186/1750-1172-7-70
Reduced elastogenesis: a clue to the arteriosclerosis and emphysematous changes in Schimke immuno-osseous dysplasia?
Abstract
Background: Arteriosclerosis and emphysema develop in individuals with Schimke immuno-osseous dysplasia (SIOD), a multisystem disorder caused by biallelic mutations in SMARCAL1 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a-like 1). However, the mechanism by which the vascular and pulmonary disease arises in SIOD remains unknown.
Methods: We reviewed the records of 65 patients with SMARCAL1 mutations. Molecular and immunohistochemical analyses were conducted on autopsy tissue from 4 SIOD patients.
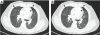
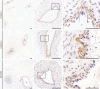
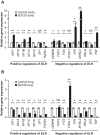
Results: Thirty-two of 63 patients had signs of arteriosclerosis and 3 of 51 had signs of emphysema. The arteriosclerosis was characterized by intimal and medial hyperplasia, smooth muscle cell hyperplasia and fragmented and disorganized elastin fibers, and the pulmonary disease was characterized by panlobular enlargement of air spaces. Consistent with a cell autonomous disorder, SMARCAL1 was expressed in arterial and lung tissue, and both the aorta and lung of SIOD patients had reduced expression of elastin and alterations in the expression of regulators of elastin gene expression.
Conclusions: This first comprehensive study of the vascular and pulmonary complications of SIOD shows that these commonly cause morbidity and mortality and might arise from impaired elastogenesis. Additionally, the effect of SMARCAL1 deficiency on elastin expression provides a model for understanding other features of SIOD.
Figures


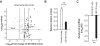
References
-
- Ehrich JH, Burchert W, Schirg E, Krull F, Offner G, Hoyer PF, Brodehl J. Steroid resistant nephrotic syndrome associated with spondyloepiphyseal dysplasia, transient ischemic attacks and lymphopenia. Clin Nephrol. 1995;43:89–95. - PubMed
-
- Schimke RN, Horton WA, King CR. Chondroitin-6-sulphaturia, defective cellular immunity, and nephrotic syndrome. Lancet. 1971;2:1088–1089. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

