Transient sodium current at subthreshold voltages: activation by EPSP waveforms
- PMID: 22998875
- PMCID: PMC3460524
- DOI: 10.1016/j.neuron.2012.08.033
Transient sodium current at subthreshold voltages: activation by EPSP waveforms
Erratum in
- Neuron. 2015 Aug 19;87(4):906
Abstract
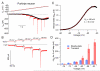
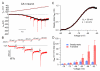
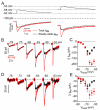
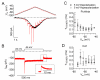
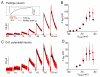
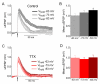
Tetrodotoxin (TTX)-sensitive sodium channels carry large transient currents during action potentials and also "persistent" sodium current, a noninactivating TTX-sensitive current present at subthreshold voltages. We examined gating of subthreshold sodium current in dissociated cerebellar Purkinje neurons and hippocampal CA1 neurons, studied at 37°C with near-physiological ionic conditions. Unexpectedly, in both cell types small voltage steps at subthreshold voltages activated a substantial component of transient sodium current as well as persistent current. Subthreshold EPSP-like waveforms also activated a large component of transient sodium current, but IPSP-like waveforms engaged primarily persistent sodium current with only a small additional transient component. Activation of transient as well as persistent sodium current at subthreshold voltages produces amplification of EPSPs that is sensitive to the rate of depolarization and can help account for the dependence of spike threshold on depolarization rate, as previously observed in vivo.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia. 2003;44:1601–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

