High-throughput screening for bioactive molecules using primary cell culture of transgenic zebrafish embryos
- PMID: 22999940
- PMCID: PMC3462279
- DOI: 10.1016/j.celrep.2012.08.015
High-throughput screening for bioactive molecules using primary cell culture of transgenic zebrafish embryos
Abstract
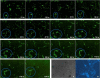
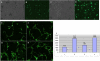
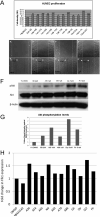
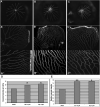
Transgenic zebrafish embryos expressing tissue-specific green fluorescent protein (GFP) can provide an unlimited supply of primary embryonic cells. Agents that promote the differentiation of these cells may be beneficial for therapeutics. We report a high-throughput approach for screening small molecules that regulate cell differentiation using lineage-specific GFP transgenic zebrafish embryonic cells. After validating several known regulators of the differentiation of endothelial and other cell types, we performed a screen for proangiogenic molecules using undifferentiated primary cells from flk1-GFP transgenic zebrafish embryos. Cells were grown in 384-well plates with 12,128 individual small molecules, and GFP expression was analyzed by means of an automated imaging system, which allowed us to screen thousands of compounds weekly. As a result, 23 molecules were confirmed to enhance angiogenesis, and 11 of them were validated to promote the proliferation of mammalian human umbilical vascular endothelial cells and induce Flk1+ cells from murine embryonic stem cells. We demonstrated the general applicability of this strategy by analyzing additional cell lineages using zebrafish expressing GFP in pancreatic, cardiac, and dopaminergic cells.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Cao Z, Jensen LD, Rouhi P, Hosaka K, Lanne T, Steffensen JF, Wahlberg E, Cao Y. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc. 2010;5:1903–1910. - PubMed
-
- Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S, Zhang L, Han Z. Hemangiopoietin promotes endothelial cell proliferation through PI-3K/Akt pathway. Cell Physiol Biochem. 2008;22:307–314. - PubMed
-
- Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. - PubMed
-
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. - PubMed
-
- Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res. 2000;86:286–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

