Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility
- PMID: 22999959
- PMCID: PMC3540263
- DOI: 10.1016/j.fertnstert.2012.08.035
Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility
Abstract
Objective: To determine the effect of infertility-linked sperm phospholipase Cζ (PLCζ) mutations on their ability to trigger oocyte Ca(2+) oscillations and development, and also to evaluate the potential therapeutic utility of wild-type, recombinant PLCζ protein for rescuing failed oocyte activation and embryo development.
Design: Test of a novel therapeutic approach to male factor infertility.
Setting: University medical school research laboratory.
Patient(s): Donated unfertilized human oocytes from follicle reduction.
Intervention(s): Microinjection of oocytes with recombinant human PLCζ protein or PLCζ cRNA and a Ca(2+)-sensitive fluorescent dye.
Main outcome measure(s): Measurement of the efficacy of mutant and wild-type PLCζ-mediated enzyme activity, oocyte Ca(2+) oscillations, activation, and early embryo development.
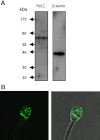
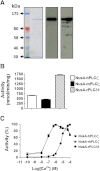
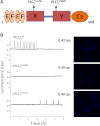
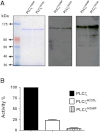
Result(s): In contrast to the wild-type protein, mutant forms of human sperm PLCζ display aberrant enzyme activity and a total failure to activate unfertilized oocytes. Subsequent microinjection of recombinant human PLCζ protein reliably triggers the characteristic pattern of cytoplasmic Ca(2+) oscillations at fertilization, which are required for normal oocyte activation and successful embryo development to the blastocyst stage.
Conclusion(s): Dysfunctional sperm PLCζ cannot trigger oocyte activation and results in male factor infertility, so a potential therapeutic approach is oocyte microinjection of active, wild-type PLCζ protein. We have demonstrated that recombinant human PLCζ can phenotypically rescue failed activation in oocytes that express dysfunctional PLCζ, and that this intervention culminates in efficient blastocyst formation.
Copyright © 2013 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ.Mol Hum Reprod. 2015 Oct;21(10):783-91. doi: 10.1093/molehr/gav042. Epub 2015 Jul 17. Mol Hum Reprod. 2015. PMID: 26187950 Free PMC article.
-
Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes.Hum Reprod. 2011 Dec;26(12):3372-87. doi: 10.1093/humrep/der336. Epub 2011 Oct 18. Hum Reprod. 2011. PMID: 22010140 Free PMC article.
-
Phospholipase C zeta (PLCζ) and male infertility: Clinical update and topical developments.Adv Biol Regul. 2016 May;61:58-67. doi: 10.1016/j.jbior.2015.11.009. Epub 2015 Nov 30. Adv Biol Regul. 2016. PMID: 26700242 Review.
-
The mammalian sperm factor phospholipase C zeta is critical for early embryo division and pregnancy in humans and mice.Hum Reprod. 2024 Jun 3;39(6):1256-1274. doi: 10.1093/humrep/deae078. Hum Reprod. 2024. PMID: 38670547 Free PMC article.
-
Increasing associations between defects in phospholipase C zeta and conditions of male infertility: not just ICSI failure?J Assist Reprod Genet. 2020 Jun;37(6):1273-1293. doi: 10.1007/s10815-020-01748-z. Epub 2020 Apr 14. J Assist Reprod Genet. 2020. PMID: 32285298 Free PMC article. Review.
Cited by
-
The fate of porcine sperm CRISP2 from the perinuclear theca before and after in vitro fertilization†.Biol Reprod. 2022 Nov 14;107(5):1242-1253. doi: 10.1093/biolre/ioac169. Biol Reprod. 2022. PMID: 36054334 Free PMC article.
-
Artificial oocyte activation with Ca2+ ionophore improves reproductive outcomes in patients with fertilization failure and poor embryo development in previous ICSI cycles.Front Endocrinol (Lausanne). 2023 Aug 11;14:1244507. doi: 10.3389/fendo.2023.1244507. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635975 Free PMC article.
-
Sperm-oocyte interplay: an overview of spermatozoon's role in oocyte activation and current perspectives in diagnosis and fertility treatment.Cell Biosci. 2021 Jan 6;11(1):4. doi: 10.1186/s13578-020-00520-1. Cell Biosci. 2021. PMID: 33407934 Free PMC article. Review.
-
Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ.Mol Hum Reprod. 2015 Oct;21(10):783-91. doi: 10.1093/molehr/gav042. Epub 2015 Jul 17. Mol Hum Reprod. 2015. PMID: 26187950 Free PMC article.
-
High rate of detected variants in male PLCZ1 and ACTL7A genes causing failed fertilization after ICSI.Hum Reprod Open. 2024 Sep 28;2024(4):hoae057. doi: 10.1093/hropen/hoae057. eCollection 2024. Hum Reprod Open. 2024. PMID: 39411542 Free PMC article.
References
-
- Kline D., Kline J.T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992;149:80–89. - PubMed
-
- Swann K., Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. Int J Dev Biol. 2008;52:585–594. - PubMed
-
- Nomikos M., Swann K., Lai F.A. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays. 2011;34:126–134. - PubMed
-
- Saunders C.M. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. - PubMed
-
- Cox L.J., Larman M.G., Saunders C.M., Hashimoto K., Swann K., Lai F.A. Sperm phospholipase Cζ from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

