Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress
- PMID: 22999971
- PMCID: PMC3489988
- DOI: 10.1016/j.neuroscience.2012.09.028
Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress
Abstract
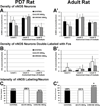
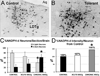
The development of tolerance to the antinociceptive effects of morphine has been associated with networks within ventrolateral periaqueductal gray (vlPAG) and separately, nitric oxide signaling. Furthermore, it is known that the mechanisms that underlie tolerance differ with age. In this study, we used a rat model of antinociceptive tolerance to morphine at two ages, postnatal day (PD) 7 and adult, to determine if changes in the vlPAG related to nitric oxide signaling produced by chronic morphine exposure were age-dependent. Three pharmacological groups were analyzed: control, acute morphine, and chronic morphine group. Either morphine (10mg/kg) or equal volume of normal saline was given subcutaneously twice daily for 6½ days. Animals were analyzed for morphine dose-response using Hot Plate test. The expression of several genes associated with nitric oxide metabolism was evaluated using rtPCR. In addition, the effect of morphine exposure on immunohistochemistry for Fos, and nNOS as well as nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) reaction at the vlPAG were measured. In both age groups acute morphine activated Fos in the vlPAG, and this effect was attenuated by chronic morphine, specifically in the vlPAG at the level of the laterodorsal tegmental nucleus (LDTg). In adults, but not PD7 rats, chronic morphine administration was associated with activation of nitric oxide function. In contrast, changes in the gene expression of PD7 rats suggested superoxide and peroxide metabolisms may be engaged. These data indicate that there is supraspinal neuroplasticity following morphine administration as early as PD7. Furthermore, oxidative stress pathways associated with chronic morphine exposure appear age-specific.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
There are no conflicts of interest.
Figures




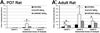
References
-
- Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. - PubMed
-
- Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW. Nitric oxide and opioid tolerance. Neuropharmacology. 1994;33:1463–1470. - PubMed
-
- Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. - PubMed
-
- Barbaresi P. Postnatal development of GABA-immunoreactive neurons and terminals in rat periaqueductal gray matter: a light and electron microscopic study. J Comp Neurol. 2010;518:2240–2260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

