Prostate cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
- PMID: 23000116
- PMCID: PMC3582194
- DOI: 10.1016/j.canep.2012.08.008
Prostate cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
Abstract
Background: The prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized screening trial demonstrated no mortality effect of screening. Here we analyze prostate cancer specific survival in PLCO and its relation to screening.
Methods: 76,693 men aged 55-74 were randomized to usual care (n = 38,350) or intervention (n = 38,343). Intervention arm men received annual prostate-specific antigen (6 years) and digital rectal exam (4 years). Men were followed for cancer diagnosis and mortality through 13 years. Medical record abstractors confirmed prostate cancer diagnoses, stage and grade. Prostate-specific survival in PLCO cases was analyzed using Kaplan-Meier analysis and proportional hazards modeling. We utilized data from the Surveillance, Epidemiology and End Results (SEER) program to compute expected survival in PLCO and compared this to observed.
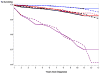
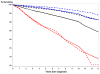
Results: There was no significant difference in prostate-specific survival rates between arms; 10 year survival rates were 94.7% (intervention, n = 4250 cases) versus 93.5% (usual care, n = 3815 cases). Within the intervention arm, cases never screened in PLCO had lower 10 year survival rates (82%) than screen detected or interval (following a negative screen) cases, both around 95.5%. The ratio of observed to expected 10 year prostate-specific death (1-survival) rates was 0.59 (95% CI: 0.51-0.68) for all PLCO cases, 0.66 (95% CI: 0.51-0.81) for Gleason 5-7 cases and 1.07 (95% CI: 0.87-1.3) for Gleason 8-10 cases.
Conclusion: Prostate cancer specific survival in PLCO was comparable across arms and significantly better than expected based on nationwide population data. How much of the better survival is due to a healthy volunteer effect and to lead-time and overdiagnosis biases is not readily determinable.
Published by Elsevier Ltd.
Conflict of interest statement
The authors have no conflicts of interest with regards to this manuscript.
Figures
References
-
- Schroder JH, Hugossan J, Roobol MJ, Tammela T, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. - PubMed
-
- Andriole GL, Crawford ED, Grubb R, Buys S, Chia D, Church TR, et al. Screening for prostate cancer, 13-year update of the results of the prostate component of the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. J Natl Cancer Inst. 2012;104:1–8. - PubMed
-
- Prorok P, Andriole GL, Bresalier RS, Buys S, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

