Deep brain photoreceptors control light-seeking behavior in zebrafish larvae
- PMID: 23000151
- PMCID: PMC3494761
- DOI: 10.1016/j.cub.2012.08.016
Deep brain photoreceptors control light-seeking behavior in zebrafish larvae
Abstract
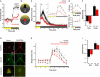
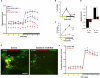
Most vertebrates process visual information using elaborately structured photosensory tissues, including the eyes and pineal. However, there is strong evidence that other tissues can detect and respond to photic stimuli. Many reports suggest that photosensitive elements exist within the brain itself and influence physiology and behavior; however, a long-standing puzzle has been the identity of the neurons and photoreceptor molecules involved. We tested whether light cues influence behavior in zebrafish larvae through deep brain photosensors. We found that larvae lacking eyes and pineal perform a simple light-seeking behavior triggered by loss of illumination ("dark photokinesis"). Neuroanatomical considerations prompted us to test orthopedia (otpa)-deficient fish, which show a profound reduction in dark photokinesis. Using targeted genetic ablations, we narrowed the photosensitive region to neurons in the preoptic area. Neurons in this region express several photoreceptive molecules, but expression of the melanopsin opn4a is selectively lost in otpa mutants, suggesting that opn4a mediates dark photokinesis. Our findings shed light on the identity and function of deep brain photoreceptors and suggest that otpa specifies an ancient population of sensory neurons that mediate behavioral responses to light.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Light perception: more than meets the eyes.Curr Biol. 2012 Nov 6;22(21):R912-4. doi: 10.1016/j.cub.2012.08.038. Curr Biol. 2012. PMID: 23137685
References
-
- Frisch K.v. Beitrage zur Physiologie der Pigmentzellen in der Fischhaut. Pflugers Archiv European Journal of Physiology. 1911;138:319–387.
-
- Adler K. Extraocular photoreception in amphibians. Photophysiology. 1976;23:275–298. - PubMed
-
- Yokoyama K, Oksche A, Darden TR, Farner DS. The sites of encephalic photoreception in photoperiodic induction of the growth of the testes in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Cell and tissue research. 1978;189:441–467. - PubMed
-
- Groos G. The comparative physiology of extraocular photoreception. Experientia. 1982;38:989–991. - PubMed
-
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell and tissue research. 1988;253:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

