Influence of apolipoprotein A-V on hepatocyte lipid droplet formation
- PMID: 23000161
- PMCID: PMC3492953
- DOI: 10.1016/j.bbrc.2012.09.065
Influence of apolipoprotein A-V on hepatocyte lipid droplet formation
Abstract
Apolipoprotein A-V (apoA-V) is postulated to modulate intra-hepatic triglyceride (TG) trafficking. Stably transfected McA-RH7777 hepatocarcinoma cells expressing human apoA-V displayed enhanced neutral lipid staining while conditioned media from these cells had 40±8% less TG than cells transfected with a control vector. To obtain homogeneous cell lines expressing different amounts of apoA-V, a strategy of clonal selection was pursued. Immunoblot analysis of two distinct apoA-V stable cell lines yielded one that expresses low amounts of apoA-V and another that expresses higher amounts. Confocal fluorescence microscopy of control cells and cells expressing low levels of apoA-V had similar numbers of lipid droplets while cells expressing higher amounts of apoA-V had twice as many lipid droplets, on average. Thus, apoA-V expression promotes lipid droplet accumulation in these cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


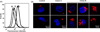
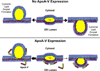
Similar articles
-
Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol.Curr Opin Lipidol. 2013 Apr;24(2):153-9. doi: 10.1097/MOL.0b013e32835c8c1a. Curr Opin Lipidol. 2013. PMID: 23241513 Free PMC article. Review.
-
Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion.J Lipid Res. 2011 Feb;52(2):237-44. doi: 10.1194/jlr.M010793. Epub 2010 Nov 26. J Lipid Res. 2011. PMID: 21115968 Free PMC article.
-
Apolipoprotein A-V association with intracellular lipid droplets.J Lipid Res. 2007 Jul;48(7):1445-50. doi: 10.1194/jlr.C700002-JLR200. Epub 2007 Apr 25. J Lipid Res. 2007. PMID: 17460328
-
ApoA-IV modulates the secretory trafficking of apoB and the size of triglyceride-rich lipoproteins.J Lipid Res. 2012 Apr;53(4):736-43. doi: 10.1194/jlr.M019992. Epub 2012 Jan 18. J Lipid Res. 2012. PMID: 22257482 Free PMC article.
-
Apolipoprotein A-V dependent modulation of plasma triacylglycerol: a puzzlement.Biochim Biophys Acta. 2012 May;1821(5):795-9. doi: 10.1016/j.bbalip.2011.12.002. Epub 2011 Dec 22. Biochim Biophys Acta. 2012. PMID: 22209939 Free PMC article. Review.
Cited by
-
Alterations in sorting and secretion of hepatic apoA5 induce hypertriglyceridemia due to short-term use of olanzapine.Front Pharmacol. 2022 Aug 12;13:935362. doi: 10.3389/fphar.2022.935362. eCollection 2022. Front Pharmacol. 2022. PMID: 36034782 Free PMC article.
-
Gene transfer of apolipoprotein A-V improves the hypertriglyceridemic phenotype of apoa5 (-/-) mice.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):474-80. doi: 10.1161/ATVBAHA.112.301078. Epub 2013 Jan 17. Arterioscler Thromb Vasc Biol. 2013. PMID: 23329134 Free PMC article.
-
Implication between Genetic Variants from APOA5 and ZPR1 and NAFLD Severity in Patients with Hypertriglyceridemia.Nutrients. 2021 Feb 8;13(2):552. doi: 10.3390/nu13020552. Nutrients. 2021. PMID: 33567543 Free PMC article.
-
Emerging evidences for the opposite role of apolipoprotein C3 and apolipoprotein A5 in lipid metabolism and coronary artery disease.Lipids Health Dis. 2019 Dec 13;18(1):220. doi: 10.1186/s12944-019-1166-5. Lipids Health Dis. 2019. PMID: 31836003 Free PMC article. Review.
-
Influence of apolipoprotein A-V on the metabolic fate of triacylglycerol.Curr Opin Lipidol. 2013 Apr;24(2):153-9. doi: 10.1097/MOL.0b013e32835c8c1a. Curr Opin Lipidol. 2013. PMID: 23241513 Free PMC article. Review.
References
-
- Johansen CT, Wang J, Lanktree MB, Cao H, McIntyre AD, Ban MR, Martins RA, Kennedy BA, Hassell RG, Visser ME, Schwartz SM, Voight BF, Elosua R, Salomaa V, O'Donnell CJ, Dallinga-Thie GM, Anand SS, Yusuf S, Huff MW, Kathiresan S, Hegele RA. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 2010;42:684–687. - PMC - PubMed
-
- Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. - PubMed
-
- Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 2001;276:44512–44520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

