Extensive degradation of RNA precursors by the exosome in wild-type cells
- PMID: 23000176
- PMCID: PMC3496076
- DOI: 10.1016/j.molcel.2012.08.018
Extensive degradation of RNA precursors by the exosome in wild-type cells
Abstract
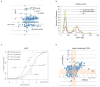
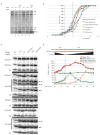
The exosome is a complex involved in the maturation of rRNA and sn-snoRNA, in the degradation of short-lived noncoding RNAs, and in the quality control of RNAs produced in mutants. It contains two catalytic subunits, Rrp6p and Dis3p, whose specific functions are not fully understood. We analyzed the transcriptome of combinations of Rrp6p and Dis3p catalytic mutants by high-resolution tiling arrays. We show that Dis3p and Rrp6p have both overlapping and specific roles in degrading distinct classes of substrates. We found that transcripts derived from more than half of intron-containing genes are degraded before splicing. Surprisingly, we also show that the exosome degrades large amounts of tRNA precursors despite the absence of processing defects. These results underscore the notion that large amounts of RNAs produced in wild-type cells are discarded before entering functional pathways and suggest that kinetic competition with degradation proofreads the efficiency and accuracy of processing.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




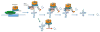
References
-
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21:87–96. - PubMed
-
- Andrulis ED, Werner J, Nazarian A, Erdjument-Bromage H, Tempst P, Lis JT. The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature. 2002;420:837–841. - PubMed
-
- Bonneau F, Basquin J, Ebert J, Lorentzen E, Conti E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell. 2009;139:547–559. - PubMed
-
- Bousquet-Antonelli C, Presutti C, Tollervey D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell. 2000;102:765–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

