Proton-coupled dynamics in lactose permease
- PMID: 23000385
- PMCID: PMC3496080
- DOI: 10.1016/j.str.2012.08.021
Proton-coupled dynamics in lactose permease
Abstract
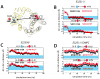
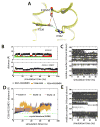
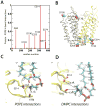
Lactose permease of Escherichia coli (LacY) catalyzes symport of a galactopyranoside and an H⁺ via an alternating access mechanism. The transition from an inward- to an outward-facing conformation of LacY involves sugar-release followed by deprotonation. Because the transition depends intimately upon the dynamics of LacY in a bilayer environment, molecular dynamics (MD) simulations may be the only means of following the accompanying structural changes in atomic detail. Here, we describe MD simulations of wild-type apo LacY in phosphatidylethanolamine (POPE) lipids that features two protonation states of the critical Glu325. While the protonated system displays configurational stability, deprotonation of Glu325 causes significant structural rearrangements that bring into proximity side chains important for H⁺ translocation and sugar binding and closes the internal cavity. Moreover, protonated LacY in phosphatidylcholine (DMPC) lipids shows that the observed dynamics are lipid-dependent. Together, the simulations describe early dynamics of the inward-to-outward transition of LacY that agree well with experimental data.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






References
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK051131/DK/NIDDK NIH HHS/United States
- GM074637/GM/NIGMS NIH HHS/United States
- GM086685/GM/NIGMS NIH HHS/United States
- P01 GM086685/GM/NIGMS NIH HHS/United States
- GM074929/GM/NIGMS NIH HHS/United States
- U54 GM074929/GM/NIGMS NIH HHS/United States
- R01 GM074637/GM/NIGMS NIH HHS/United States
- R56 DK051131/DK/NIDDK NIH HHS/United States
- GM073210/GM/NIGMS NIH HHS/United States
- DK069463/DK/NIDDK NIH HHS/United States
- DK051131/DK/NIDDK NIH HHS/United States
- P50 GM073210/GM/NIGMS NIH HHS/United States
- R56 DK069463/DK/NIDDK NIH HHS/United States
- R01 DK069463/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

