Dual modulation of cyclooxygenase and CYP epoxygenase metabolism and acute vascular inflammation in mice
- PMID: 23000418
- PMCID: PMC3549041
- DOI: 10.1016/j.prostaglandins.2012.09.003
Dual modulation of cyclooxygenase and CYP epoxygenase metabolism and acute vascular inflammation in mice
Abstract
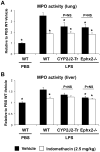
Cyclooxygenase (COX)-derived prostaglandins and cytochrome P450 (CYP) epoxygenase-derived epoxyeicosatrienoic acids are important regulators of inflammation; however, functional interactions between these pathways in the regulation of vascular inflammation in vivo have not been studied. We investigated the relative and additive effects of endothelial CYP2J2 overexpression (Tie2-CYP2J2-Tr), global sEH disruption (Ephx2(-/-)), and pharmacologic COX inhibition with indomethacin on the acute vascular inflammatory response to endotoxin in mice. Compared to vehicle-treated wild-type C57BL/6 controls, induction of myeloperoxidase (MPO) activity in lung and liver was similarly attenuated in Tie2-CYP2J2-Tr mice, Ephx2(-/-) mice and wild-type mice treated with moderate dose indomethacin. Dual modulation of both pathways, however, did not produce an additive anti-inflammatory effect. These findings demonstrate that both COX and CYP epoxygenase-mediated eicosanoid metabolism are important regulators of the acute vascular inflammatory response in vivo, and suggest that the anti-inflammatory effects of modulating each pathway may be mediated, at least in part, by overlapping mechanisms.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–95. - PubMed
-
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–45. - PubMed
-
- Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

