Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects
- PMID: 23000587
- PMCID: PMC3467406
- DOI: 10.4142/jvs.2012.13.3.299
Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects
Abstract
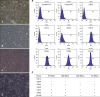
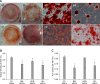
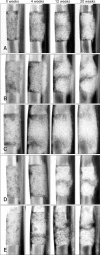
Alternative sources of mesenchymal stem cells (MSCs) for replacing bone marrow (BM) have been extensively investigated in the field of bone tissue engineering. The purpose of this study was to compare the osteogenic potential of canine MSCs derived from adipose tissue (AT), BM, umbilical cord blood (UCB), and Wharton's jelly (WJ) using in vitro culture techniques and in vivo orthotopic implantation assays. After canine MSCs were isolated from various tissues, the proliferation and osteogenic potential along with vascular endothelial growth factor (VEGF) production were measured and compared in vitro. For the in vivo assay, MSCs derived from each type of tissue were mixed with β-tricalcium phosphate and implanted into segmental bone defects in dogs. Among the different types of MSCs, AT-MSCs had a higher proliferation potential and BM-MSCs produced the most VEGF. AT-MSCs and UCB-MSCs showed greater in vitro osteogenic potential compared to the other cells. Radiographic and histological analyses showed that all tested MSCs had similar osteogenic capacities, and the level of new bone formation was much higher with implants containing MSCs than cell-free implants. These results indicate that AT-MSCs, UCB-MSCs, and WJ-MSCs can potentially be used in place of BM-MSCs for clinical bone engineering procedures.
Figures




References
-
- Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–629. - PubMed
-
- Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85:1927–1935. - PubMed
-
- Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218:237–245. - PubMed
-
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. - PubMed
-
- Bianco P, Kuznetsov SA, Riminucci M, Robey PG. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

