Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 underlies the wiry leaf syndrome
- PMID: 23001036
- PMCID: PMC3480288
- DOI: 10.1105/tpc.112.100222
Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 underlies the wiry leaf syndrome
Abstract
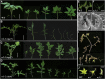
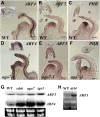
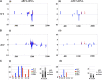
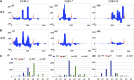
Interfering with small RNA production is a common strategy of plant viruses. A unique class of small RNAs that require microRNA and short interfering (siRNA) biogenesis for their production is termed trans-acting short interfering RNAs (ta-siRNAs). Tomato (Solanum lycopersicum) wiry mutants represent a class of phenotype that mimics viral infection symptoms, including shoestring leaves that lack leaf blade expansion. Here, we show that four WIRY genes are involved in siRNA biogenesis, and in their corresponding mutants, levels of ta-siRNAs that regulate AUXIN RESPONSE FACTOR3 (ARF3) and ARF4 are reduced, while levels of their target ARFs are elevated. Reducing activity of both ARF3 and ARF4 can rescue the wiry leaf lamina, and increased activity of either can phenocopy wiry leaves. Thus, a failure to negatively regulate these ARFs underlies tomato shoestring leaves. Overexpression of these ARFs in Arabidopsis thaliana, tobacco (Nicotiana tabacum), and potato (Solanum tuberosum) failed to produce wiry leaves, suggesting that the dramatic response in tomato is exceptional. As negative regulation of orthologs of these ARFs by ta-siRNA is common to land plants, we propose that ta-siRNA levels serve as universal sensors for interference with small RNA biogenesis, and changes in their levels direct species-specific responses.
Figures


Comment in
-
Why wiry? Tomato mutants reveal connections among small RNAs, auxin response factors, virus infection, and leaf morphology.Plant Cell. 2012 Sep;24(9):3486. doi: 10.1105/tpc.112.240911. Epub 2012 Sep 21. Plant Cell. 2012. PMID: 23001038 Free PMC article. No abstract available.
References
-
- Adenot X., Elmayan T., Lauressergues D., Boutet S., Bouché N., Gasciolli V., Vaucheret H. (2006). DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr. Biol. 16: 927–932 - PubMed
-
- Allen E., Howell M.D. (2010). miRNAs in the biogenesis of trans-acting siRNAs in higher plants. Semin. Cell Dev. Biol. 21: 798–804 - PubMed
-
- Allen E., Xie Z., Gustafson A.M., Carrington J.C. (2005). MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

