Propofol at clinically relevant concentrations increases neuronal differentiation but is not toxic to hippocampal neural precursor cells in vitro
- PMID: 23001052
- PMCID: PMC3483886
- DOI: 10.1097/ALN.0b013e31826f8d86
Propofol at clinically relevant concentrations increases neuronal differentiation but is not toxic to hippocampal neural precursor cells in vitro
Abstract
Background: Propofol in the early postnatal period has been shown to cause brain cell death. One proposed mechanism for cognitive dysfunction after anesthesia is alteration of neural stem cell function and neurogenesis. We examined the effect of propofol on neural precursor or stem cells (NPCs) grown in vitro.
Methods: Hippocampal-derived NPCs from postnatal day 2 rats were exposed to propofol or Diprivan. NPCs were then analyzed for bromodeoxyuridine incorporation to measure proliferation. Cell death was measured by lactate dehydrogenase release. Immunocytochemistry was used to evaluate the expression of neuronal and glial markers in differentiating NPCs exposed to propofol.
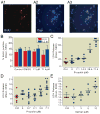
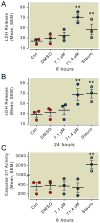
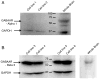
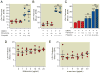
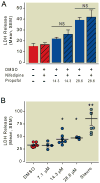
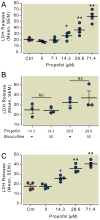
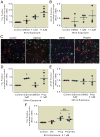
Results: Propofol dose dependently increases the release of lactate dehydrogenase from NPCs under both proliferating and differentiating conditions at supraclinical concentrations (more than 7.1 µM). Both Diprivan and propofol had the same effect on NPCs. Propofol-mediated release of lactate dehydrogenase is not inhibited by blocking the γ-aminobutyric acid type A receptor or extracellular calcium influx and is not mediated by caspase-3/7. Direct γ-aminobutyric acid type A receptor activation did not have the same effect. In differentiating NPCs, 6 h of propofol at 2.1 µM increased the number neurons but not glial cells 4 days later. Increased neuronal differentiation was not blocked by bicuculline.
Conclusions: Only supraclinical concentrations of propofol or Diprivan kill NPCs in culture by a non-γ-aminobutyric acid type A, noncaspase-3 mechanism. Clinically relevant doses of propofol increase neuronal fate choice by a non-γ-aminobutyric acid type A mechanism.
Figures
References
-
- Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–4. - PubMed
-
- Bercker S, Bert B, Bittigau P, Felderhoff-Muser U, Buhrer C, Ikonomidou C, Weise M, Kaisers UX, Kerner T. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–7. - PubMed
-
- Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7:249–71. - PubMed

