Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression
- PMID: 23001085
- PMCID: PMC4162482
- DOI: 10.1097/SLA.0b013e31826d90bb
Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression
Abstract
Objective: To minimize maintenance immunosuppression in upper-extremity transplantation to favor the risk-benefit balance of this procedure.
Background: Despite favorable outcomes, broad clinical application of reconstructive transplantation is limited by the risks and side effects of multidrug immunosuppression. We present our experience with upper-extremity transplantation under a novel, donor bone marrow (BM) cell-based treatment protocol ("Pittsburgh protocol").
Methods: Between March 2009 and September 2010, 5 patients received a bilateral hand (n = 2), a bilateral hand/forearm (n = 1), or a unilateral (n = 2) hand transplant. Patients were treated with alemtuzumab and methylprednisolone for induction, followed by tacrolimus monotherapy. On day 14, patients received an infusion of donor BM cells isolated from 9 vertebral bodies. Comprehensive follow-up included functional evaluation, imaging, and immunomonitoring.
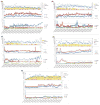
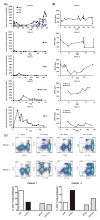
Results: All patients are maintained on tacrolimus monotherapy with trough levels ranging between 4 and 12 ng/mL. Skin rejections were infrequent and reversible. Patients demonstrated sustained improvements in motor function and sensory return correlating with time after transplantation and level of amputation. Side effects included transient increase in serum creatinine, hyperglycemia managed with oral hypoglycemics, minor wound infection, and hyperuricemia but no infections. Immunomonitoring revealed transient moderate levels of donor-specific antibodies, adequate immunocompetence, and no peripheral blood chimerism. Imaging demonstrated patent vessels with only mild luminal narrowing/occlusion in 1 case. Protocol skin biopsies showed absent or minimal perivascular cellular infiltrates.
Conclusions: Our data suggest that this BM cell-based treatment protocol is safe, is well tolerated, and allows upper-extremity transplantation using low-dose tacrolimus monotherapy.
Trial registration: ClinicalTrials.gov NCT00722280.
Conflict of interest statement
Disclosure: The authors declare no conflicts of interest.
Figures

References
-
- Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2010;90:1590–1594. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

