Annotating MYC status with 89Zr-transferrin imaging
- PMID: 23001181
- PMCID: PMC3521603
- DOI: 10.1038/nm.2935
Annotating MYC status with 89Zr-transferrin imaging
Abstract
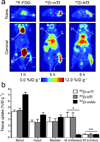
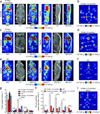
A noninvasive technology that quantitatively measures the activity of oncogenic signaling pathways could have a broad impact on cancer diagnosis and treatment with targeted therapies. Here we describe the development of (89)Zr-desferrioxamine-labeled transferrin ((89)Zr-transferrin), a new positron emission tomography (PET) radiotracer that binds the transferrin receptor 1 (TFRC, CD71) with high avidity. The use of (89)Zr-transferrin produces high-contrast PET images that quantitatively reflect treatment-induced changes in MYC-regulated TFRC expression in a MYC-driven prostate cancer xenograft model. Moreover, (89)Zr-transferrin imaging can detect the in situ development of prostate cancer in a transgenic MYC prostate cancer model, as well as in prostatic intraepithelial neoplasia (PIN) before histological or anatomic evidence of invasive cancer. These preclinical data establish (89)Zr-transferrin as a sensitive tool for noninvasive measurement of oncogene-driven TFRC expression in prostate and potentially other cancers, with prospective near-term clinical application.
Figures

References
-
- Neckers LM, Trepel JB. Transferrin receptor expression and the control of cell growth. Cancer Invest. 1986;4:461–470. - PubMed
-
- Richardson DR. Therapeutic potential of iron chelators in cancer therapy. Adv Exp Med Biol. 2002;509:231–249. - PubMed
-
- Taetle R, Honeysett JM, Trowbridge I. Effects of anti-transferrin receptor antibodies on growth of normal and malignant myeloid cells. Int J Cancer. 1983;32:343–349. - PubMed
-
- Weiner RE. The mechanism of 67Ga localization in malignant disease. Nucl Med Biol. 1996;23:745–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

