A fully humanized transgenic mouse model of Huntington disease
- PMID: 23001568
- PMCID: PMC3606012
- DOI: 10.1093/hmg/dds397
A fully humanized transgenic mouse model of Huntington disease
Abstract
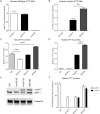
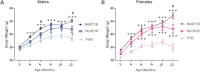
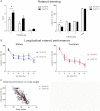
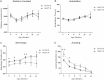
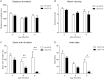
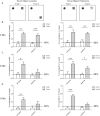
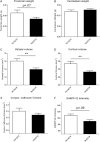
Silencing the mutant huntingtin gene (muHTT) is a direct and simple therapeutic strategy for the treatment of Huntington disease (HD) in principle. However, targeting the HD mutation presents challenges because it is an expansion of a common genetic element (a CAG tract) that is found throughout the genome. Moreover, the HTT protein is important for neuronal health throughout life, and silencing strategies that also reduce the wild-type HTT allele may not be well tolerated during the long-term treatment of HD. Several HTT silencing strategies are in development that target genetic sites in HTT that are outside of the CAG expansion, including HD mutation-linked single-nucleotide polymorphisms and the HTT promoter. Preclinical testing of these genetic therapies has required the development of a new mouse model of HD that carries these human-specific genetic targets. To generate a fully humanized mouse model of HD, we have cross-bred BACHD and YAC18 on the Hdh(-/-) background. The resulting line, Hu97/18, is the first murine model of HD that fully genetically recapitulates human HD having two human HTT genes, no mouse Hdh genes and heterozygosity of the HD mutation. We find that Hu97/18 mice display many of the behavioral changes associated with HD including motor, psychiatric and cognitive deficits, as well as canonical neuropathological abnormalities. This mouse line will be useful for gaining additional insights into the disease mechanisms of HD as well as for testing genetic therapies targeting human HTT.
Figures



Similar articles
-
A novel humanized mouse model of Huntington disease for preclinical development of therapeutics targeting mutant huntingtin alleles.Hum Mol Genet. 2017 Mar 15;26(6):1115-1132. doi: 10.1093/hmg/ddx021. Hum Mol Genet. 2017. PMID: 28104789
-
Striatal synaptic dysfunction and hippocampal plasticity deficits in the Hu97/18 mouse model of Huntington disease.PLoS One. 2014 Apr 11;9(4):e94562. doi: 10.1371/journal.pone.0094562. eCollection 2014. PLoS One. 2014. PMID: 24728353 Free PMC article.
-
In vivo evaluation of candidate allele-specific mutant huntingtin gene silencing antisense oligonucleotides.Mol Ther. 2014 Dec;22(12):2093-2106. doi: 10.1038/mt.2014.153. Epub 2014 Aug 7. Mol Ther. 2014. PMID: 25101598 Free PMC article.
-
Aberrant palmitoylation in Huntington disease.Biochem Soc Trans. 2015 Apr;43(2):205-10. doi: 10.1042/BST20140242. Biochem Soc Trans. 2015. PMID: 25849918 Review.
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
Cited by
-
Allele-specific silencing of mutant huntingtin in rodent brain and human stem cells.PLoS One. 2014 Jun 13;9(6):e99341. doi: 10.1371/journal.pone.0099341. eCollection 2014. PLoS One. 2014. PMID: 24926995 Free PMC article.
-
Evidences for Mutant Huntingtin Inducing Musculoskeletal and Brain Growth Impairments via Disturbing Testosterone Biosynthesis in Male Huntington Disease Animals.Cells. 2022 Nov 25;11(23):3779. doi: 10.3390/cells11233779. Cells. 2022. PMID: 36497038 Free PMC article.
-
The effects of huntingtin-lowering: what do we know so far?Degener Neurol Neuromuscul Dis. 2019 Mar 8;9:3-17. doi: 10.2147/DNND.S163808. eCollection 2019. Degener Neurol Neuromuscul Dis. 2019. PMID: 30881191 Free PMC article. Review.
-
Small molecule splicing modifiers with systemic HTT-lowering activity.Nat Commun. 2021 Dec 15;12(1):7299. doi: 10.1038/s41467-021-27157-z. Nat Commun. 2021. PMID: 34911927 Free PMC article.
-
Oligonucleotide-based strategies to combat polyglutamine diseases.Nucleic Acids Res. 2014 Jun;42(11):6787-810. doi: 10.1093/nar/gku385. Epub 2014 May 21. Nucleic Acids Res. 2014. PMID: 24848018 Free PMC article. Review.
References
-
- Ross C.A., Tabrizi S.J. Huntington's disease, from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. - PubMed
-
- Hawkins A.K., Ho A., Hayden M.R. Lessons from predictive testing for Huntington disease: 25 years on. J. Med. Genet. 2011;48:649–650. - PubMed
-
- Yamamoto A., Lucas J.J., Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. - PubMed
-
- Diaz-Hernandez M., Torres-Peraza J., Salvatori-Abarca A., Moran M.A., Gomez-Ramos P., Alberch J., Lucas J.J. Full motor recovery despite striatal neuron loss and formation of irreversible amyloid-like inclusions in a conditional mouse model of Huntington's disease. J. Neurosci. 2005;25:9773–9781. - PMC - PubMed
-
- Harper S.Q., Staber P.D., He X., Eliason S.L., Martins I.H., Mao Q., Yang L., Kotin R.M., Paulson H.L., Davidson B.L. From the Cover: RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl Acad. Sci. USA. 2005;102:5820–5825. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

