In vivo diffusion tensor MRI of the human heart: reproducibility of breath-hold and navigator-based approaches
- PMID: 23001828
- PMCID: PMC3864770
- DOI: 10.1002/mrm.24488
In vivo diffusion tensor MRI of the human heart: reproducibility of breath-hold and navigator-based approaches
Erratum in
- Magn Reson Med. 2014 Aug;72(2):599
Abstract
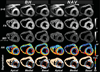
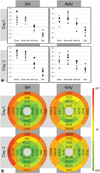
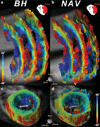
The aim of this study was to implement a quantitative in vivo cardiac diffusion tensor imaging (DTI) technique that was robust, reproducible, and feasible to perform in patients with cardiovascular disease. A stimulated-echo single-shot echo-planar imaging (EPI) sequence with zonal excitation and parallel imaging was implemented, together with a novel modification of the prospective navigator (NAV) technique combined with a biofeedback mechanism. Ten volunteers were scanned on two different days, each time with both multiple breath-hold (MBH) and NAV multislice protocols. Fractional anisotropy (FA), mean diffusivity (MD), and helix angle (HA) fiber maps were created. Comparison of initial and repeat scans showed good reproducibility for both MBH and NAV techniques for FA (P > 0.22), MD (P > 0.15), and HA (P > 0.28). Comparison of MBH and NAV FA (FAMBHday1 = 0.60 ± 0.04, FANAVday1 = 0.60 ± 0.03, P = 0.57) and MD (MDMBHday1 = 0.8 ± 0.2 × 10(-3) mm(2) /s, MDNAVday1 = 0.9 ± 0.2 × 10(-3) mm(2) /s, P = 0.07) values showed no significant differences, while HA values (HAMBHday1Endo = 22 ± 10°, HAMBHday1Mid-Endo = 20 ± 6°, HAMBHday1Mid-Epi = -1 ± 6°, HAMBHday1Epi = -17 ± 6°, HANAVday1Endo = 7 ± 7°, HANAVday1Mid-Endo = 13 ± 8°, HANAVday1Mid-Epi = -2 ± 7°, HANAVday1Epi = -14 ± 6°) were significantly different. The scan duration was 20% longer with the NAV approach. Currently, the MBH approach is the more robust in normal volunteers. While the NAV technique still requires resolution of some bulk motion sensitivity issues, these preliminary experiments show its potential for in vivo clinical cardiac diffusion tensor imaging and for delivering high-resolution in vivo 3D DTI tractography of the heart.
Keywords: cardiac diffusion tensor imaging; cardiac diffusion-weighted imaging; cardiovascular magnetic resonance imaging; prospective navigators.
© 2012 Wiley Periodicals, Inc.
Figures




References
-
- Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. - PubMed
-
- Streeter DD, Jr, Hanna WT. Engineering mechanics for successive states in canine left ventricular myocardium. II. Fiber angle and sarcomere length. Circ Res. 1973;33:656–664. - PubMed
-
- Edelman RR, Gaa J, Wedeen VJ, Loh E, Hare JM, Prasad P, Li W. In vivo measurement of water diffusion in the human heart. Magn Reson Med. 1994;32:423–428. - PubMed
-
- Reese TG, Weisskoff RM, Smith RN, Rosen BR, Dinsmore RE, Wedeen VJ. Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn Reson Med. 1995;34:786–791. - PubMed
-
- Tseng WY, Reese TG, Weisskoff RM, Brady TJ, Wedeen VJ. Myocardial fiber shortening in humans: initial results of MR imaging. Radiology. 2000;216:128–139. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

