Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow
- PMID: 23002006
- PMCID: PMC3535508
- DOI: 10.1002/art.37709
Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow
Abstract
Objective: B cell depletion therapy ameliorates rheumatoid arthritis by mechanisms that are incompletely understood. Arthritis flare in tumor necrosis factor (TNF)-transgenic mice is associated with efferent lymph node (LN) "collapse," triggered by B cell translocation into lymphatic spaces and decreased lymphatic drainage. The aim of this study was to examine whether the efficacy of B cell depletion therapy is associated with restoration of lymphatic drainage due to removal of obstructing nodal B cells.
Methods: We used contrast-enhanced magnetic resonance imaging, indocyanine green near-infrared imaging, and intravital immunofluorescence imaging to longitudinally assess synovitis, lymphatic flow, and cell migration in lymphatic vessels in TNF-transgenic mice. We conducted tests to determine whether the efficacy of B cell depletion therapy is associated with restoration of lymphatic draining and cell egress from arthritic joints.
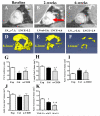
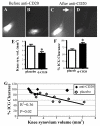
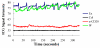
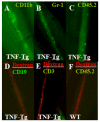
Results: Unlike active lymphatics to normal and prearthritic knees, afferent lymphatic vessels to collapsed LNs in inflamed knees do not pulse. Intravital immunofluorescence imaging demonstrated that CD11b+ monocyte/macrophages in lymphatic vessels afferent to expanding LNs travel at high velocity (mean±SD 186±37 μm/second), while these cells are stationary in lymphatic vessels afferent to collapsed popliteal LNs. B cell depletion therapy for arthritis flares in TNF-transgenic mice significantly decreased knee synovium volume (by 50% from the baseline level) and significantly increased lymphatic clearance compared with placebo (P<0.05). This increased lymphatic drainage restored macrophage egress from inflamed joints without recovery of the lymphatic pulse.
Conclusion: These results support a novel mechanism in which B cell depletion therapy for joint arthritis flares lessens inflammation by increasing lymphatic drainage and subsequent migration of cells and cytokines from the synovial space.
Copyright © 2013 by the American College of Rheumatology.
Figures
References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. - PubMed
-
- Maini RN, Elliott MJ, Brennan FM, Williams RO, Chu CQ, Paleolog E, et al. Monoclonal anti-TNF alpha antibody as a probe of pathogenesis and therapy of rheumatoid disease. Immunol Rev. 1995;144:195–223. - PubMed
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343(22):1594–602. - PubMed
-
- Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337(3):141–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

