Activation of KCNN3/SK3/K(Ca)2.3 channels attenuates enhanced calcium influx and inflammatory cytokine production in activated microglia
- PMID: 23002008
- PMCID: PMC3799773
- DOI: 10.1002/glia.22419
Activation of KCNN3/SK3/K(Ca)2.3 channels attenuates enhanced calcium influx and inflammatory cytokine production in activated microglia
Abstract
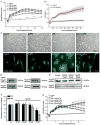
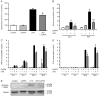
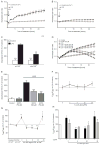
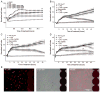
In neurons, small-conductance calcium-activated potassium (KCNN/SK/K(Ca)2) channels maintain calcium homeostasis after N-methyl-D-aspartate (NMDA) receptor activation, thereby preventing excitotoxic neuronal death. So far, little is known about the function of KCNN/SK/K(Ca)2 channels in non-neuronal cells, such as microglial cells. In this study, we addressed the question whether KCNN/SK/K(Ca)2 channels activation affected inflammatory responses of primary mouse microglial cells upon lipopolysaccharide (LPS) stimulation. We found that N-cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA), a positive pharmacological activator of KCNN/SK/K(Ca)2 channels, significantly reduced LPS-stimulated activation of microglia in a concentration-dependent manner. The general KCNN/SK/K(Ca)2 channel blocker apamin reverted these effects of CyPPA on microglial proliferation. Since calcium plays a central role in microglial activation, we further addressed whether KCNN/SK/K(Ca)2 channel activation affected the changes of intracellular calcium levels, [Ca(2+)](i), in microglial cells. Our data show that LPS-induced elevation of [Ca(2+)](i) was attenuated following activation of KCNN2/3/K(Ca)2.2/K(Ca)2.3 channels by CyPPA. Furthermore, CyPPA reduced downstream events including tumor necrosis factor alpha and interleukin 6 cytokine production and nitric oxide release in activated microglia. Further, we applied specific peptide inhibitors of the KCNN/SK/K(Ca)2 channel subtypes to identify which particular channel subtype mediated the observed anti-inflammatory effects. Only inhibitory peptides targeting KCNN3/SK3/K(Ca)2.3 channels, but not KCNN2/SK2/K(Ca)2.2 channel inhibition, reversed the CyPPA-effects on LPS-induced microglial proliferation. These findings revealed that KCNN3/SK3/K(Ca)2.3 channels can modulate the LPS-induced inflammatory responses in microglial cells. Thus, KCNN3/SK3/K(Ca)2.3 channels may serve as a therapeutic target for reducing microglial activity and related inflammatory responses in the central nervous system.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Bordey A, Spencer DD. Chemokine modulation of high-conductance Ca2+-sensitive K+ currents in microglia from human hippocampi. Eur J Neurosci. 2003;18:2893–2898. - PubMed
-
- Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci. 2000;12:2049–2058. - PubMed
-
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia—New concepts. Brain Res Rev. 2007;53:344–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

