U.S. Food and Drug Administration Approval: peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma
- PMID: 23002124
- PMCID: PMC3481898
- DOI: 10.1634/theoncologist.2012-0123
U.S. Food and Drug Administration Approval: peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma
Abstract
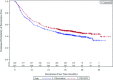
On March 29, 2011, the U.S. Food and Drug Administration approved peginterferon alfa-2b (PEG-IFN) (Sylatron™; Schering Corporation, Kenilworth, NJ) for the adjuvant treatment of melanoma patients with microscopic or gross nodal involvement following definitive surgical resection including complete lymphadenectomy. The approval was based on a single, open-label, multicenter trial enrolling 1,256 patients. After surgical resection, patients were randomized (1:1) to either PEG-IFN or observation for 5 years. PEG-IFN, 6 μg/kg per week, was administered s.c. for eight doses, followed by 3 μg/kg per week for up to 252 weeks. Stratification factors included microscopic or gross nodal involvement, number of positive nodes, Breslow thickness, ulceration, sex, and study center. Patients were assessed for recurrence by the investigators based on physical examination every 3 months for 2 years and every 6 months thereafter. The relapse-free survival (RFS) interval, the primary efficacy endpoint, was significantly longer in PEG-IFN-treated patients. The median RFS times were 34.8 months and 25.5 months, respectively. There was no statistically significant difference in the overall survival time. The most common (>60%) grade 1-4 adverse reactions were fatigue, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST), pyrexia, headache, anorexia, myalgia, nausea, chills, and injection site reactions. The most common serious adverse reactions were fatigue, increased ALT and AST, and pyrexia. Thirty-three percent of patients receiving PEG-IFN discontinued treatment as a result of adverse reactions. Five deaths were reported within 30 days of the last treatment dose, two resulting from cardiovascular disease considered as possibly related to treatment.
Conflict of interest statement
Section Editors:
Reviewer “A”: Novartis, Merck, Bristol-Myers Squibb, Prometheus (C/A); Prometheus (H)
Reviewer “B”: Merck (C/A), (H)
Figures
Comment in
-
Pegylated interferon for the adjuvant treatment of melanoma: FDA approved, but what is its role?Oncologist. 2012;17(10):1223-4. doi: 10.1634/theoncologist.2012-0368. Epub 2012 Sep 21. Oncologist. 2012. PMID: 23002125 Free PMC article.
References
-
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. - PubMed
-
- Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. - PubMed
-
- Janku F, Kurzrock R. Adjuvant interferon in high-risk melanoma: End of the era? J Clin Oncol. 2010;28:e15–e16. - PubMed
-
- Zhou Q, Zhang XS. Adjuvant interferon therapy for malignant melanoma: The debate. Chin J Cancer. 2010;29:907–913. - PubMed
-
- Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical