Mechanical tension modulates local and global vesicle dynamics in neurons
- PMID: 23002399
- PMCID: PMC3445628
- DOI: 10.1007/s12195-012-0223-1
Mechanical tension modulates local and global vesicle dynamics in neurons
Abstract
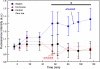
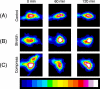
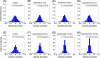
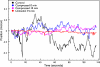
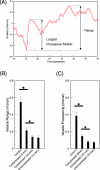
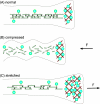
Growing experimental evidence suggests that mechanical tension plays a significant role in determining the growth, guidance, and function of neurons. Mechanical tension in axons contributes to neurotransmitter clustering at the Drosophila neuromuscular junction (NMJ) and is actively regulated by neurons both in vitro and in vivo. In this work, we applied mechanical strain on in vivo Drosophila neurons and in vitro Aplysia neurons and studied their vesicle dynamics by live-imaging. Our experiments show that mechanical stretch modulates the dynamics of vesicles in two different model systems: (1) The global accumulation of synaptic vesicles (SV) at the Drosophila NMJ and (2) the local motion of individual large dense core vesicles (LDCV) in Aplysia neurites. Specifically, a sustained stretch results in enhanced SV accumulation in the Drosophila NMJ. This increased SV accumulation occurs in the absence of extracellular Ca(2+), plateaus after approximately 50 min, and persists for at least 30 min after stretch is reduced. On the other hand, mechanical compression in Aplysia neurites immediately disrupts LDCV motion, leading to decreased range and processivity. This impairment of LDCV motion persists for at least 15 min after tension is restored. These results show that mechanical stretch modulates both local and global vesicle dynamics and strengthens the notion that tension serves a role in regulating neuronal function.
Figures

References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–41.
-
- Ayali A. The function of mechanical tension in neuronal and network development. Integr. Biol. 2010;2(4):178–82. - PubMed
-
- Bernal R, Pullarkat PA, Melo F. Mechanical properties of axons. Phys Rev Lett. 2007;99(1):018301. - PubMed
-
- Bixby JL. Ultrastructural observations on synapse elimination in neonatal rabbit skeletal muscle. J Neurocytol. 1981;10(1):81–100. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

