Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis
- PMID: 23002959
- PMCID: PMC3585469
- DOI: 10.1089/scd.2012.0395
Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis
Abstract
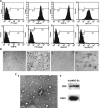
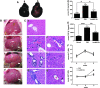
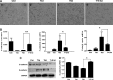
Mesenchymal stem cells (MSCs) have been considered as an attractive tool for the therapy of diseases. Exosomes excreted from MSCs can reduce myocardial ischemia/reperfusion damage and protect against acute tubular injury. However, whether MSC-derived exosomes can relieve liver fibrosis and its mechanism remain unknown. Previous work showed that human umbilical cord-MSCs (hucMSCs) transplanted into acutely injured and fibrotic livers could restore liver function and improve liver fibrosis. In this study, it was found that transplantation of exosomes derived from hucMSC (hucMSC-Ex) reduced the surface fibrous capsules and got their textures soft, alleviated hepatic inflammation and collagen deposition in carbon tetrachloride (CCl4)-induced fibrotic liver. hucMSC-Ex also significantly recovered serum aspartate aminotransferase (AST) activity, decreased collagen type I and III, transforming growth factor (TGF)-β1 and phosphorylation Smad2 expression in vivo. In further experiments, we found that epithelial-to-mesenchymal transition (EMT)-associated markers E-cadherin-positive cells increased and N-cadherin- and vimentin-positive cells decreased after hucMSC-Ex transplantation. Furthermore, the human liver cell line HL7702 underwent typical EMT after induction with recombinant human TGF-β1, and then hucMSC-Ex treatment reversed spindle-shaped and EMT-associated markers expression in vitro. Taken together, these results suggest that hucMSC-Ex could ameliorate CCl4-induced liver fibrosis by inhibiting EMT and protecting hepatocytes. This provides a novel approach for the treatment of fibrotic liver disease.
Figures



References
-
- Snowdon VK. Fallowfield JA. Models and mechanisms of fibrosis resolution. Alcohol Clin Exp Res. 2011;35:794–799. - PubMed
-
- Domitrović R. Jakovac H. Effects of standardized bilberry fruit extract on resolution of CCl4-induced liver fibrosis in mice. Food Chem Toxicol. 2011;49:848–854. - PubMed
-
- Zeisberg M. Yang C. Martino M. Duncan MB. Rieder F. Tanjore H. Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

