Transporter targeted gatifloxacin prodrugs: synthesis, permeability, and topical ocular delivery
- PMID: 23003105
- PMCID: PMC3926802
- DOI: 10.1021/mp300245r
Transporter targeted gatifloxacin prodrugs: synthesis, permeability, and topical ocular delivery
Abstract
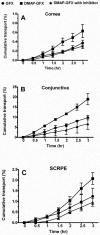
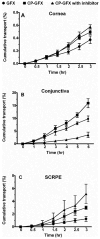
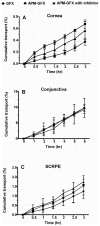
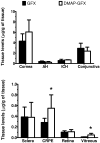
In this work, we aim to design and synthesize prodrugs of gatifloxacin targeting organic cation transporter (OCT), monocarboxylate transporter (MCT), and ATB (0, +) transporters and to identify a prodrug with enhanced delivery to the back of the eye. Dimethylamino-propyl, carboxy-propyl, and amino-propyl(2-methyl) derivatives of gatifloxacin (GFX), DMAP-GFX, CP-GFX, and APM-GFX, were designed and synthesized to target OCT, MCT, and ATB (0, +) transporters, respectively. An LC-MS method was developed to analyze drug and prodrug levels in various studies. Solubility and log D (pH 7.4) were measured for prodrugs and the parent drug. The permeability of the prodrugs was determined in the cornea, conjunctiva, and sclera-choroid-retinal pigment epitheluim (SCRPE) and compared with gatifloxacin using an Ussing chamber assembly. Permeability mechanisms were elucidated by determining the transport in the presence of transporter specific inhibitors. 1-Methyl-4-phenylpyridinium iodide (MPP+), nicotinic acid sodium salt, and α-methyl-DL-tryptophan were used to inhibit OCT, MCT, and ATB (0, +) transporters, respectively. A prodrug selected based on in vitro studies was administered as an eye drop to pigmented rabbits, and the delivery to various eye tissues including vitreous humor was compared with gatifloxacin dosing. DMAP-GFX exhibited 12.8-fold greater solubility than GFX. All prodrugs were more lipophilic, with the measured log D (pH 7.4) values ranging from 0.05 to 1.04, when compared to GFX (log D: -1.15). DMAP-GFX showed 1.4-, 1.8-, and 1.9-fold improvement in permeability across the cornea, conjunctiva, and SCRPE when compared to GFX. Moreover, it exhibited reduced permeability in the presence of MPP+ (competitive inhibitor of OCT), indicating OCT-mediated transport. CP-GFX showed 1.2-, 2.3-, and 2.5-fold improvement in permeability across the cornea, conjunctiva, and SCRPE, respectively. In the presence of nicotinic acid (competitive inhibitor of MCT), the permeability of CP-GFX was reduced across the conjunctiva. However, the cornea and SCRPE permeability of CP-GFX was not affected by nicotinic acid. APM-GFX did not show any improvement in permeability when compared to GFX across the cornea, conjunctiva, and SCRPE. Based on solubility and permeability, DMAP-GFX was selected for in vivo studies. DMAP-GFX showed 3.6- and 1.95-fold higher levels in vitreous humor and CRPE compared to that of GFX at 1 h after topical dosing. In vivo conversion of DMAP-GFX prodrug to GFX was quantified in tissues isolated at 1 h after dosing. The parent drug-to-prodrug ratio was 8, 70, 24, 21, 29, 13, 55, and 60% in the cornea, conjunctiva, iris-ciliary body, aqueous humor, sclera, CRPE, retina, and vitreous humor, respectively. In conclusion, DMAP-GFX prodrug enhanced solubility, log D, as well as OCT mediated delivery of gatifloxacin to the back of the eye.
Figures



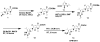
Similar articles
-
Advances in the use of prodrugs for drug delivery to the eye.Expert Opin Drug Deliv. 2017 Jan;14(1):49-63. doi: 10.1080/17425247.2016.1208649. Epub 2016 Jul 21. Expert Opin Drug Deliv. 2017. PMID: 27441817 Free PMC article. Review.
-
Nanosized dendritic polyguanidilyated translocators for enhanced solubility, permeability, and delivery of gatifloxacin.Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5804-16. doi: 10.1167/iovs.10-5388. Epub 2010 May 19. Invest Ophthalmol Vis Sci. 2010. PMID: 20484584
-
Immunohistochemical and functional characterization of peptide, organic cation, neutral and basic amino acid, and monocarboxylate drug transporters in human ocular tissues.Drug Metab Dispos. 2013 Feb;41(2):466-74. doi: 10.1124/dmd.112.045674. Epub 2012 Nov 20. Drug Metab Dispos. 2013. Retraction in: Drug Metab Dispos. 2015 Feb;43(2):234. doi: 10.1124/dmd.114.045674err. PMID: 23169611 Free PMC article. Retracted.
-
Hydrophilic prodrug approach for reduced pigment binding and enhanced transscleral retinal delivery of celecoxib.Mol Pharm. 2012 Mar 5;9(3):605-14. doi: 10.1021/mp2005164. Epub 2012 Feb 8. Mol Pharm. 2012. Retraction in: Mol Pharm. 2015 Jul 6;12(7):2558. doi: 10.1021/acs.molpharmaceut.5b00366. PMID: 22256989 Free PMC article. Retracted.
-
A Minireview: Usefulness of Transporter-Targeted Prodrugs in Enhancing Membrane Permeability.J Pharm Sci. 2016 Sep;105(9):2515-2526. doi: 10.1016/j.xphs.2016.05.012. Epub 2016 Jun 16. J Pharm Sci. 2016. PMID: 27321236 Review.
Cited by
-
Novel Eye Drop Delivery Systems: Advance on Formulation Design Strategies Targeting Anterior and Posterior Segments of the Eye.Pharmaceutics. 2022 May 27;14(6):1150. doi: 10.3390/pharmaceutics14061150. Pharmaceutics. 2022. PMID: 35745723 Free PMC article. Review.
-
Post-traumatic endophthalmitis prophylaxis with oral ciprofloxacin in comparison to intravenous cephazolin/gentamicin.J Res Med Sci. 2018 Nov 28;23:98. doi: 10.4103/jrms.JRMS_384_18. eCollection 2018. J Res Med Sci. 2018. PMID: 30595706 Free PMC article.
-
Advances in the use of prodrugs for drug delivery to the eye.Expert Opin Drug Deliv. 2017 Jan;14(1):49-63. doi: 10.1080/17425247.2016.1208649. Epub 2016 Jul 21. Expert Opin Drug Deliv. 2017. PMID: 27441817 Free PMC article. Review.
-
Prodrug approach to improve absorption of prednisolone.Int J Pharm. 2015 Jun 20;487(1-2):242-9. doi: 10.1016/j.ijpharm.2015.04.029. Epub 2015 Apr 15. Int J Pharm. 2015. PMID: 25888804 Free PMC article.
References
-
- Williams A, Sloan FA, Lee PP. Longitudinal rates of cataract surgery. Arch Ophthalmol. 2006;124(9):1308–14. - PubMed
-
- Lalwani GA, Flynn HW, Jr, Scott IU, Quinn CM, Berrocal AM, Davis JL, Murray TG, Smiddy WE, Miller D. Acute-onset endophthalmitis after clear corneal cataract surgery (1996- 2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115(3):473–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

