Effects of 405 nm diode laser on titanium oxide bleaching activation
- PMID: 23003121
- PMCID: PMC3487294
- DOI: 10.1089/pho.2012.3273
Effects of 405 nm diode laser on titanium oxide bleaching activation
Abstract
Objective: The objective of this study was to evaluate the effects of a 405 nm diode laser on bleaching reaction of H(2)O(2) and VL-TiO(2) on methylene blue (MB) dye.
Background data: Visible light activating titanium dioxide photocatalyst (VL-TiO(2)) may improve efficacy of hydrogen peroxide (H(2)O(2)) bleaching agents used in dentistry while contributing to their safety by lowering the required concentration of peroxide.
Methods: The experimental solution was prepared with H(2)O(2), VL-TiO(2), MB, and pure water. The final concentration of H(2)O(2) was 3.5% and that of MB was 10 ppm. The experimental solution of 3 mL in a quartz cell was irradiated by a 405 nm diode laser with various powers, duty cycles, and pulse durations for 7 min.
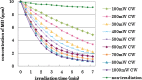
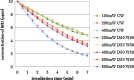
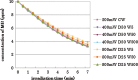
Results: In all irradiation conditions, the increase in laser irradiation time gradually decreased the MB concentration. Irradiation by higher output power showed more reduction of MB concentration. Pulse durations as short as 5 ms with duty cycle reduced to 25% did not affect the degree of the reduction in MB concentration compared with continuous wave irradiation at the same average output power.
Conclusions: It was concluded that using 405 nm diode laser, the bleaching effects of VL-TiO(2) depended upon the irradiation time and the average output power, regardless of pulse duration or duty cycle.
Figures

Similar articles
-
Effects of light sources and visible light-activated titanium dioxide photocatalyst on bleaching.Dent Mater J. 2009 Nov;28(6):693-9. doi: 10.4012/dmj.28.693. Dent Mater J. 2009. PMID: 20019420
-
Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst.Dent Mater J. 2011;30(5):723-9. doi: 10.4012/dmj.2010-210. Epub 2011 Sep 23. Dent Mater J. 2011. PMID: 21946494
-
Exploring the efficacy of laser-assisted in-office tooth bleaching: A study on varied irradiation times and power settings utilizing a diode laser (445 nm).J Photochem Photobiol B. 2024 Aug;257:112970. doi: 10.1016/j.jphotobiol.2024.112970. Epub 2024 Jun 28. J Photochem Photobiol B. 2024. PMID: 38955079
-
Analysis of shade, temperature and hydrogen peroxide concentration during dental bleaching: in vitro study with the KTP and diode lasers.Lasers Med Sci. 2013 Jan;28(1):1-6. doi: 10.1007/s10103-011-1037-4. Epub 2011 Dec 24. Lasers Med Sci. 2013. PMID: 22198709
-
Laser teeth bleaching: evaluation of eventual side effects on enamel and the pulp and the efficiency in vitro and in vivo.ScientificWorldJournal. 2015;2015:835405. doi: 10.1155/2015/835405. Epub 2015 Mar 22. ScientificWorldJournal. 2015. PMID: 25874258 Free PMC article. Review.
Cited by
-
Novel Experimental In-Office Bleaching Gels Containing Co-Doped Titanium Dioxide Nanoparticles.Nanomaterials (Basel). 2022 Aug 30;12(17):2995. doi: 10.3390/nano12172995. Nanomaterials (Basel). 2022. PMID: 36080033 Free PMC article.
-
In Vitro Assessment of SWEEPS and Antimicrobial Photodynamic Therapy Alone or in Combination for Eradicating Enterococcus faecalis Biofilm in Root Canals.Pharmaceutics. 2023 Nov 15;15(11):2628. doi: 10.3390/pharmaceutics15112628. Pharmaceutics. 2023. PMID: 38004605 Free PMC article.
-
A novel approach for in-office tooth bleaching with 6% H2O2/TiO_N and LED/laser system-a controlled, triple-blinded, randomized clinical trial.Lasers Med Sci. 2016 Apr;31(3):437-44. doi: 10.1007/s10103-016-1866-2. Epub 2016 Jan 21. Lasers Med Sci. 2016. PMID: 26796706 Clinical Trial.
-
Nitrogen-Doped Titanium Dioxide Mixed with Calcium Peroxide and Methylcellulose for Dental Bleaching under Visible Light Activation.Int J Mol Sci. 2021 Apr 4;22(7):3759. doi: 10.3390/ijms22073759. Int J Mol Sci. 2021. PMID: 33916642 Free PMC article.
-
Titanium dioxide nanotubes in a hydrogen peroxide-based bleaching agent: physicochemical properties and effectiveness of dental bleaching under the influence of a poliwave led light activation.Clin Oral Investig. 2023 Apr;27(4):1745-1755. doi: 10.1007/s00784-022-04802-5. Epub 2022 Nov 28. Clin Oral Investig. 2023. PMID: 36441269
References
-
- ADA Council on Scientific Affairs. Tooth whitening/bleaching: treatment considerations for dentists and their patients. Chicago: American Dental Association; 2009.
-
- Sulieman M.A. An overview of bleaching techniques: chemistry, safety and efficacy. Periodontol 2000. 2008;48:148–169. - PubMed
-
- Matis B.A. Cochran M.A. Eckert G. Review of the effectiveness of various tooth whitening systems. Oper. Dent. 2009;34:230–235. - PubMed
-
- Davidi M.P. Hadad A. Weiss E.I. Domb A. Mizrahi B. Sterer N. The effect of a mild increase in temperature on tooth bleaching. Quintessence Int. 2008;39:771–775. - PubMed
-
- Buchalla W. Attin T. External bleaching therapy with activation by heat, light or laser – A systematic review. Dent. Mater. 2007;23:586–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

