Long-term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non-small-cell lung cancer
- PMID: 23004347
- PMCID: PMC7657241
- DOI: 10.1111/cas.12028
Long-term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non-small-cell lung cancer
Abstract
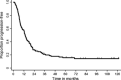
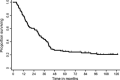
Concurrent chemoradiotherapy is the standard treatment for unresectable stage III non-small cell lung cancer (NSCLC). The long-term feasibility and efficacy of vinorelbine and cisplatin with concurrent thoracic radiotherapy were investigated. Eighteen patients received cisplatin (80 mg/m(2)) on day 1 and vinorelbine (20 mg/m(2) in level 1, and 25 mg/m(2) in level 2) on days 1 and 8 every 4 weeks for four cycles in a phase I trial. Ninety-three patients received the same chemotherapy regimen except for the fixed vinorelbine (20 mg/m(2)) dosage and consolidation therapy with docetaxel (60 mg/m(2), every 3 weeks). The thoracic radiotherapy consisted of a single dose of 2 Gy once daily to a total dose of 60 Gy. A total of 111 patients were analyzed in the present study: male/female, 91/20; median age, 60 years; stage IIIA/IIIB, 50/61; and squamous/non-squamous histology, 26/85. The 3-, 5-, and 7-year overall survival rates (95% CI) were 43.2% (33.9-52.2), 25.2% (17.6-33.5), and 23.2% (15.8-31.4), respectively. The median progression-free survival and median survival time (95% CI) were 13.5 (10.1-16.7) months and 30.0 (24.3-38.8) months, respectively. Four patients (4%) experienced Grade 5 pulmonary toxicities from 4.4 to 9.4 months after the start of treatment. In conclusion, approximately 15% of patients with unresectable stage III NSCLC could be cured with chemoradiotherapy without severe late toxicities after 10 months of follow-up. Although based on the data from highly selected population participated in phase I and phase II trial, this analysis would strengthen and confirm the previous reports concerning concurrent chemoradiotherapy with third generation cytotoxic agents.
© 2012 Japanese Cancer Association.
Figures
References
-
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997; 111: 1710–7. - PubMed
-
- Meerbeeck V. Staging of non‐small cell lung cancer: consensus, controversies and challenges. Lung Cancer 2001; 34: S95–107. - PubMed
-
- Jett JR, Scott WJ, Rivera MP, Sause WT. Guidelines on treatment of stage IIIB non‐small cell lung cancer. Chest 2003; 123: 221S–5S. - PubMed
-
- Furuse K, Fukuoka M, Kawahara M et al Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol 1999; 17: 2692–9. - PubMed
-
- Fournel P, Robinet G, Thomas P et al Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐Saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 Study. J Clin Oncol 2005; 23: 5910–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical