Evidence for radical-mediated catalysis by HppE: a study using cyclopropyl and methylenecyclopropyl substrate analogues
- PMID: 23006053
- PMCID: PMC3463719
- DOI: 10.1021/ja3078126
Evidence for radical-mediated catalysis by HppE: a study using cyclopropyl and methylenecyclopropyl substrate analogues
Abstract
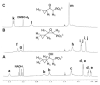
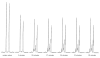
(S)-2-Hydroxypropylphosphonic acid epoxidase (HppE) is an unusual mononuclear iron enzyme that catalyzes the oxidative epoxidation of (S)-2-hydroxypropylphosphonic acid ((S)-HPP) in the biosynthesis of the antibiotic fosfomycin. HppE also recognizes (R)-2-hydroxypropylphosphonic acid ((R)-HPP) as a substrate and converts it to 2-oxo-propylphosphonic acid. To probe the mechanisms of these HppE-catalyzed oxidations, cyclopropyl- and methylenecyclopropyl-containing compounds were synthesized and studied as radical clock substrate analogues. Enzymatic assays indicated that the (S)- and (R)-isomers of the cyclopropyl-containing analogues were efficiently converted to epoxide and ketone products by HppE, respectively. In contrast, the ultrafast methylenecyclopropyl-containing probe inactivated HppE, consistent with a rapid radical-triggered ring-opening process that leads to enzyme inactivation. Taken together, these findings provide, for the first time, experimental evidence for the involvement of a C2-centered radical intermediate with a lifetime on the order of nanoseconds in the HppE-catalyzed oxidation of (R)-HPP.
Figures



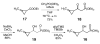


Similar articles
-
Mechanistic consequences of chiral radical clock probes: analysis of the mononuclear non-heme iron enzyme HppE with 2-hydroxy-3-methylenecyclopropyl radical clock substrates.J Am Chem Soc. 2014 Feb 26;136(8):2944-7. doi: 10.1021/ja4100035. Epub 2014 Feb 13. J Am Chem Soc. 2014. PMID: 24512048 Free PMC article.
-
Reaction of HppE with substrate analogues: evidence for carbon-phosphorus bond cleavage by a carbocation rearrangement.J Am Chem Soc. 2013 Jun 5;135(22):8153-6. doi: 10.1021/ja403441x. Epub 2013 May 23. J Am Chem Soc. 2013. PMID: 23672451 Free PMC article.
-
Structural basis of regiospecificity of a mononuclear iron enzyme in antibiotic fosfomycin biosynthesis.J Am Chem Soc. 2011 Jul 27;133(29):11262-9. doi: 10.1021/ja2025728. Epub 2011 Jun 30. J Am Chem Soc. 2011. PMID: 21682308 Free PMC article.
-
Adenosylation reactions catalyzed by the radical S-adenosylmethionine superfamily enzymes.Curr Opin Chem Biol. 2020 Apr;55:86-95. doi: 10.1016/j.cbpa.2020.01.007. Epub 2020 Feb 18. Curr Opin Chem Biol. 2020. PMID: 32086168 Review.
-
Radical SAM enzymes involved in the biosynthesis of purine-based natural products.Biochim Biophys Acta. 2012 Nov;1824(11):1245-53. doi: 10.1016/j.bbapap.2012.07.014. Epub 2012 Aug 3. Biochim Biophys Acta. 2012. PMID: 22902275 Free PMC article. Review.
Cited by
-
Mechanistic consequences of chiral radical clock probes: analysis of the mononuclear non-heme iron enzyme HppE with 2-hydroxy-3-methylenecyclopropyl radical clock substrates.J Am Chem Soc. 2014 Feb 26;136(8):2944-7. doi: 10.1021/ja4100035. Epub 2014 Feb 13. J Am Chem Soc. 2014. PMID: 24512048 Free PMC article.
-
Biochemistry: Positive and radical.Nature. 2013 Apr 4;496(7443):34-5. doi: 10.1038/496034a. Nature. 2013. PMID: 23552937 No abstract available.
-
Reaction of HppE with substrate analogues: evidence for carbon-phosphorus bond cleavage by a carbocation rearrangement.J Am Chem Soc. 2013 Jun 5;135(22):8153-6. doi: 10.1021/ja403441x. Epub 2013 May 23. J Am Chem Soc. 2013. PMID: 23672451 Free PMC article.
-
Mechanistic studies of an unprecedented enzyme-catalysed 1,2-phosphono-migration reaction.Nature. 2013 Apr 4;496(7443):114-8. doi: 10.1038/nature11998. Nature. 2013. PMID: 23552950 Free PMC article.
-
Probing the mechanism of cyanobacterial aldehyde decarbonylase using a cyclopropyl aldehyde.J Am Chem Soc. 2013 Apr 10;135(14):5234-7. doi: 10.1021/ja3115949. Epub 2013 Apr 2. J Am Chem Soc. 2013. PMID: 23514600 Free PMC article.
References
-
- Raz R. Clin. Microbiol. Infect. 2012;18:4–7. - PubMed
-
- Hidaka T, Goda M, Kuzuyama T, Takei N, Hidaka M, Seto H. Mol. Gen. Genet. 1995;249:274–280. - PubMed
-
- Liu P, Murakami K, Seki T, He X, Yeung SM, Kuzuyama T, Seto H, Liu H.-w. J. Am. Chem. Soc. 2001;123:4619–4620. - PubMed
-
- Liu P, Liu A, Yan F, Wolfe MD, Lipscomb JD, Liu H.-w. Biochemistry. 2003;42:11577–11586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

