Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity
- PMID: 23006327
- PMCID: PMC3461929
- DOI: 10.1172/JCI64837
Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity
Abstract
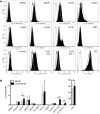
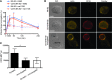
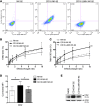
The Fc receptor on NK cells, FcγRIIIA (CD16), has been extensively studied for its role in mediating antibody-dependent cellular cytotoxicity (ADCC). A homozygous missense mutation in CD16 (encoding a L66H substitution) is associated with severe herpesvirus infections in rare patients. Here, we identified a new patient with this CD16 mutation and compared the patient's NK cells to those of the originally reported patient. Patients with the L66H mutation had intact ADCC, but deficient spontaneous NK cell cytotoxicity and decreased surface expression of CD2, a coactivation receptor. Mechanistic studies in a human NK cell line, NK-92, demonstrated that CD16 expression correlated with CD2 surface levels and enabled killing of a melanoma cell line typically resistant to CD16-deficient NK-92 cells. An association between CD16 and CD2 was identified biochemically and at the immunological synapse, which elicited CD16 signaling after CD2 engagement. Stable expression of CD16 L66H in NK-92 cells recapitulated the patient phenotype, abrogating association of CD16 with CD2 as well as CD16 signaling after CD2 ligation. Thus, CD16 serves a role in NK cell-mediated spontaneous cytotoxicity through a specific association with CD2 and represents a potential mechanism underlying a human congenital immunodeficiency.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

