Identification of resistant carboxylesterase alleles in Culex pipiens complex via PCR-RFLP
- PMID: 23006470
- PMCID: PMC3480951
- DOI: 10.1186/1756-3305-5-209
Identification of resistant carboxylesterase alleles in Culex pipiens complex via PCR-RFLP
Abstract
Background: Carboxylesterase overproduction is a frequently observed resistance mechanism of insects to organophosphate insecticides. As a major transmitter of human diseases, mosquitoes in the Culex pipiens complex have evolved 13 carboxylesterase alleles (Ester) that confer organophosphate resistance. Six alleles, Ester(B1), Ester², Ester⁸, Ester⁹, Ester(B10), and Ester¹¹, have been observed in field populations in China, sometimes co-existing in one population. To differentiate the carboxylesterase alleles found in these field populations, PCR-RFLP was designed for use in resistance monitoring.
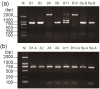
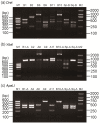
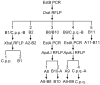
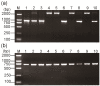
Results: Based on the DNA sequences of resistant and nonresistant carboxylesterase alleles, Ester B alleles were first amplified with PCR-specific primers and then digested with the restriction enzyme DraI. In this step, Ester² and Ester¹¹ were differentiated from the other Ester alleles. When the other Ester B alleles were digested with the restriction enzyme XbaI, Ester(B1) and the susceptible C. p. pallens Ester were screened out. Ester⁸ and Ester⁹ were differentiated from Ester(B10) and the susceptible C. p. quinquefasciatus esterase allele, respectively, by amplifying and digesting the Ester A alleles with the restriction enzyme ApaLI. The effectiveness of the custom-designed PCR-RFLP was verified in two field mosquito populations.
Conclusions: A PCR-RFLP based approach was developed to differentiate carboxylesterase alleles in Culex pipiens complex mosquitoes. These processes may be useful in monitoring the evolutionary dynamics of known carboxylesterase alleles as well as in the identification of new alleles in field populations.
Figures



References
-
- Zhao TY, Lu BL. Biosystematics of Culex pipiens complex in China. Insect Sci. 1995;2:1–8. doi: 10.1111/j.1744-7917.1995.tb00016.x. - DOI
-
- Oppenoorth FJ. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut GA, Gilbert LI, editor. Pergamon, New York; 1985. Biochemistry and genetics of insecticide resistance; pp. 731–773. Insect Control, vol.12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

