Dietary iodide controls its own absorption through post-transcriptional regulation of the intestinal Na+/I- symporter
- PMID: 23006481
- PMCID: PMC3530113
- DOI: 10.1113/jphysiol.2012.241307
Dietary iodide controls its own absorption through post-transcriptional regulation of the intestinal Na+/I- symporter
Abstract
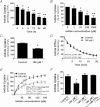
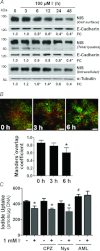
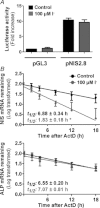
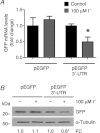
Dietary I(-) absorption in the gastrointestinal tract is the first step in I(-) metabolism. Given that I(-) is an essential constituent of the thyroid hormones, its concentrating mechanism is of significant physiological importance. We recently described the expression of the Na(+)/I(-) symporter (NIS) on the apical surface of the intestinal epithelium as a central component of the I(-) absorption system and reported reduced intestinal NIS expression in response to an I(-)-rich diet in vivo. Here, we evaluated the mechanism involved in the regulation of NIS expression by I(-) itself in enterocytes. Excess I(-) reduced NIS-mediated I(-) uptake in IEC-6 cells in a dose- and time-dependent fashion, which was correlated with a reduction of NIS expression at the plasma membrane. Perchlorate, a competitive inhibitor of NIS, prevented these effects, indicating that an increase in intracellular I(-) regulates NIS. Iodide induced rapid intracellular recruitment of plasma membrane NIS molecules and NIS protein degradation. Lower NIS mRNA levels were detected in response to I(-) treatment, although no transcriptional effect was observed. Interestingly, I(-) decreased NIS mRNA stability, affecting NIS translation. Heterologous green fluorescent protein-based reporter constructs revealed a significant repressive effect of the I(-)-targeting NIS mRNA 3 untranslated region. In conclusion, excess I(-) downregulates NIS expression in enterocytes by virtue of a complex mechanism. Our data suggest that I(-) regulates intestinal NIS mRNA expression at the post-transcriptional level as part of an autoregulatory effect of I(-) on its own metabolism.
Figures


Similar articles
-
The Na+/I- symporter mediates active iodide uptake in the intestine.Am J Physiol Cell Physiol. 2009 Apr;296(4):C654-62. doi: 10.1152/ajpcell.00509.2008. Epub 2008 Dec 3. Am J Physiol Cell Physiol. 2009. PMID: 19052257 Free PMC article.
-
Dietary I(-) absorption: expression and regulation of the Na(+)/I(-) symporter in the intestine.Vitam Horm. 2015;98:1-31. doi: 10.1016/bs.vh.2014.12.002. Epub 2015 Feb 27. Vitam Horm. 2015. PMID: 25817864 Review.
-
Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein.Endocrinology. 1999 Aug;140(8):3404-10. doi: 10.1210/endo.140.8.6893. Endocrinology. 1999. PMID: 10433193
-
Excess iodide downregulates Na(+)/I(-) symporter gene transcription through activation of PI3K/Akt pathway.Mol Cell Endocrinol. 2016 May 5;426:73-90. doi: 10.1016/j.mce.2016.02.006. Epub 2016 Feb 9. Mol Cell Endocrinol. 2016. PMID: 26872612
-
Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid.Endocrinology. 2009 Mar;150(3):1084-90. doi: 10.1210/en.2008-1437. Epub 2009 Feb 5. Endocrinology. 2009. PMID: 19196800 Free PMC article. Review.
Cited by
-
Nutritional iodine status and obesity.Thyroid Res. 2021 Nov 27;14(1):25. doi: 10.1186/s13044-021-00116-y. Thyroid Res. 2021. PMID: 34838106 Free PMC article. Review.
-
Iodide transport: implications for health and disease.Int J Pediatr Endocrinol. 2014;2014(1):8. doi: 10.1186/1687-9856-2014-8. Epub 2014 May 30. Int J Pediatr Endocrinol. 2014. PMID: 25009573 Free PMC article.
-
Asn441 plays a key role in folding and function of the Na+/I- symporter (NIS).FASEB J. 2013 Aug;27(8):3229-38. doi: 10.1096/fj.13-229138. Epub 2013 May 6. FASEB J. 2013. PMID: 23650190 Free PMC article.
-
The master role of polarized NIS expression in regulating iodine metabolism in the human body.Arch Endocrinol Metab. 2023 Mar 10;67(2):256-261. doi: 10.20945/2359-3997000000583. Arch Endocrinol Metab. 2023. PMID: 36913678 Free PMC article.
-
Implications of Na+/I- Symporter Transport to the Plasma Membrane for Thyroid Hormonogenesis and Radioiodide Therapy.J Endocr Soc. 2018 Dec 5;3(1):222-234. doi: 10.1210/js.2018-00100. eCollection 2019 Jan 1. J Endocr Soc. 2018. PMID: 30620007 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

