Sericin cream reduces pruritus in hemodialysis patients: a randomized, double-blind, placebo-controlled experimental study
- PMID: 23006933
- PMCID: PMC3472272
- DOI: 10.1186/1471-2369-13-119
Sericin cream reduces pruritus in hemodialysis patients: a randomized, double-blind, placebo-controlled experimental study
Abstract
Background: Uremic pruritus (UP) is a significant complication in ESRD patients and substantially impairs their quality of life. UP is considered to be a skin manifestation of chronic inflammation. Because sericin can suppress the release of pro-inflammatory cytokines, the purpose of this study was to investigate the short-term safety and efficacy of sericin cream for treating UP in hemodialysis patients.
Methods: This study used a double-blind design to investigate the effects of random topical administration of sericin cream and cream base (placebo) on either the right or left extremities of hemodialysis patients for 6 weeks. Skin hydration, irritation and pigmentation were evaluated every 2 weeks using Skin Diagnostic SD27. The visual analog scale for itching was also evaluated every 2 weeks, and the Kidney Disease Quality of Life Short Form was performed on the day of each patient's enrollment and after 6 weeks of treatment.
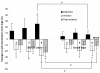
Results: Fifty dialysis patients were enrolled, 47 of which completed the study. The hydration of the skin of the patients' extremities increased significantly after administration of sericin cream; significant differences were found between sericin treatment and control after 6 weeks of treatment (p = 0.041 for arms and p = 0.022 for legs, respectively). Moreover, a significant difference was also found in skin irritation between the two treatments (p = 0.013 for arms and p = 0.027 for legs, respectively). At the end of the study, the skin pigmentation level was significantly reduced on both the arms (p = 0.032) and legs (p = 0.021) of the sericin-treated side compared with the side treated with cream base. The mean itching score decreased significantly from moderate to severe at the time of enrollment to mild pruritus after 6 weeks of treatment (p = 0.002). A better quality of life was found in all domains tested although statistically significant differences before and after treatment was found only in the patients' pain scores, the effect of kidney disease on daily life, sleep quality and symptoms or problems related to kidney disease.
Conclusions: We conclude that sericin cream has a high potential for reducing UP in hemodialysis patients.The trial registration number of this study is ISRCTN16019033; its public title is "sericin cream reduces pruritus in hemodialysis patients".
Figures


References
-
- Mettang M, Weisshaar E. Pruritus: control of itch in patients undergoing dialysis. Skin Therapy Lett. 2010;15(2):1–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

