Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response
- PMID: 23006971
- PMCID: PMC3660063
- DOI: 10.18632/oncotarget.652
Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response
Abstract
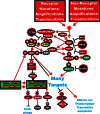
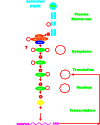
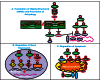
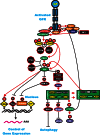
The Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades are often activated by genetic alterations in upstream signaling molecules such as receptor tyrosine kinases (RTK). Certain components of these pathways, RAS, NF1, BRAF, MEK1, DUSP5, PP2A, PIK3CA, PIK3R1, PIK3R4, PIK3R5, IRS4, AKT, NFKB1, MTOR, PTEN, TSC1, and TSC2 may also be activated/inactivated by mutations or epigenetic silencing. Upstream mutations in one signaling pathway or even in downstream components of the same pathway can alter the sensitivity of the cells to certain small molecule inhibitors. These pathways have profound effects on proliferative, apoptotic and differentiation pathways. Dysregulation of components of these cascades can contribute to: resistance to other pathway inhibitors, chemotherapeutic drug resistance, premature aging as well as other diseases. This review will first describe these pathways and discuss how genetic mutations and epigenetic alterations can result in resistance to various inhibitors.
Figures
References
-
- Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3:192–222. - PMC - PubMed
-
- Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: Rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. - PMC - PubMed
-
- McCubrey JA, Steelman LS, Kempf CR, Chappell W, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra M, Bäsecke J, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Cocco L, Martelli AM. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011;226:2762–2781. - PubMed
-
- Little AS, Smith PD, Cook SJ. Mechanisms of acquired resistance to ERK1/2 pathway inhibitors. Oncogene. 2012 May 7; doi: 10.1038/onc.2012.160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

