Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning
- PMID: 23007029
- PMCID: PMC3529181
- DOI: 10.1038/pr.2012.124
Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning
Abstract
Background: Preconditioning of neonatal mice with nonlethal hypoxia (HPC) protects the brain from hypoxic-ischemic (HI) injury. Overexpression of human glutathione peroxidase 1 (GPx1), which normally protects the developing murine brain from HI injury, reverses HPC protection, suggesting that a certain threshold of hydrogen peroxide concentration is required for activation of HPC signaling.
Methods: Activation (phosphorylation) of extracellular-regulated kinase (ERK) 1/2 and Akt, and induction of hypoxia-inducible factor (HIF)-1α were assessed in the cortex, one of the main structures affected by HI and protected by HPC, at different time points after reoxygenation in wild-type (WT) and GPx1-overexpressing animals.
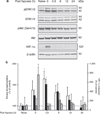
Results: GPx1 overexpression prevented both the global and nuclear increase in activated ERK at 0.5 h after HPC and caused a significant decrease in phospho-ERK (pERK)/ERK levels at 24 h after HPC. In contrast, HIF-1α induction at the end of hypoxia was unaffected by GPx1 overexpression. In the cortex of preconditioned WT animals, enhanced pERK staining was primarily observed in neurons and to a lower extent in astrocytes and endothelial cells, with a nuclear prominence.
Conclusion: Aberrant activation of ERK probably explains the paradoxical reversal of HPC protection by GPx1 overexpression. The results identify hydrogen peroxide as an important mediator of neuroprotective ERK signaling.
Figures



Similar articles
-
Hypoxic preconditioning protection is eliminated in HIF-1α knockout mice subjected to neonatal hypoxia-ischemia.Pediatr Res. 2014 Jul;76(1):46-53. doi: 10.1038/pr.2014.53. Epub 2014 Apr 8. Pediatr Res. 2014. PMID: 24713818 Free PMC article.
-
Alteration in Downstream Hypoxia Gene Signaling in Neonatal Glutathione Peroxidase Overexpressing Mouse Brain after Hypoxia-Ischemia.Dev Neurosci. 2015;37(4-5):398-406. doi: 10.1159/000375369. Epub 2015 Mar 17. Dev Neurosci. 2015. PMID: 25792071 Free PMC article.
-
[Role of hypoxia-inducible factor-1alpha in the prevention of cardiomyocyte injury induced by hypoxic preconditioning].Sheng Li Xue Bao. 2004 Oct 25;56(5):609-14. Sheng Li Xue Bao. 2004. PMID: 15497042 Chinese.
-
Extracellular signal-regulated kinase 2 has duality in function between neuronal and astrocyte expression following neonatal hypoxic-ischaemic cerebral injury.J Physiol. 2018 Dec;596(23):6043-6062. doi: 10.1113/JP275649. Epub 2018 Jul 11. J Physiol. 2018. PMID: 29873394 Free PMC article.
-
Hypoxia preconditioning protects neuronal cells against traumatic brain injury through stimulation of glucose transport mediated by HIF-1α/GLUTs signaling pathway in rat.Neurosurg Rev. 2021 Feb;44(1):411-422. doi: 10.1007/s10143-019-01228-8. Epub 2020 Jan 2. Neurosurg Rev. 2021. PMID: 31897883 Free PMC article.
Cited by
-
Role of recurrent hypoxia-ischemia in preterm white matter injury severity.PLoS One. 2014 Nov 12;9(11):e112800. doi: 10.1371/journal.pone.0112800. eCollection 2014. PLoS One. 2014. PMID: 25390897 Free PMC article.
-
CUEDC2 modulates cardiomyocyte oxidative capacity by regulating GPX1 stability.EMBO Mol Med. 2016 Jul 1;8(7):813-29. doi: 10.15252/emmm.201506010. Print 2016 Jul. EMBO Mol Med. 2016. PMID: 27286733 Free PMC article.
-
Hypoxic preconditioning protection is eliminated in HIF-1α knockout mice subjected to neonatal hypoxia-ischemia.Pediatr Res. 2014 Jul;76(1):46-53. doi: 10.1038/pr.2014.53. Epub 2014 Apr 8. Pediatr Res. 2014. PMID: 24713818 Free PMC article.
-
Repetitive ischemic preconditioning attenuates inflammatory reaction and brain damage after focal cerebral ischemia in rats: involvement of PI3K/Akt and ERK1/2 signaling pathway.J Mol Neurosci. 2015 Apr;55(4):912-22. doi: 10.1007/s12031-014-0446-9. Epub 2014 Oct 22. J Mol Neurosci. 2015. PMID: 25338292
-
Alteration in Downstream Hypoxia Gene Signaling in Neonatal Glutathione Peroxidase Overexpressing Mouse Brain after Hypoxia-Ischemia.Dev Neurosci. 2015;37(4-5):398-406. doi: 10.1159/000375369. Epub 2015 Mar 17. Dev Neurosci. 2015. PMID: 25792071 Free PMC article.
References
-
- Sheldon RA, Aminoff A, Lee CL, Christen S, Ferriero DM. Hypoxic preconditioning reverses protection after neonatal hypoxia-ischemia in glutathione peroxidase transgenic murine brain. Pediatr Res. 2007;61:666–670. - PubMed
-
- Fullerton HJ, Ditelberg JS, Chen SF, et al. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44:357–364. - PubMed
-
- Sheldon RA, Jiang X, Francisco C, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004;56:656–662. - PubMed
-
- Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res. 1998;53:333–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

