Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis
- PMID: 23007134
- PMCID: PMC3810270
- DOI: 10.1038/labinvest.2012.121
Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis
Abstract
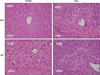
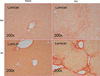
Lumican, an extracellular matrix proteoglycan was previously shown to be upregulated with increasing severity of nonalcoholic steatohepatitis (NASH). Although lumican is involved in collagen fibrillogenesis in extra-hepatic tissues, little is known about the role of lumican in hepatic disease. We therefore determined lumican expression in etiologies other than clinical NASH. Our results indicated that lumican is upregulated in clinical samples of hepatitis C virus infection, in experimental rodent models of chronic and acute liver injury and could additionally be induced in vitro in response to the pro-fibrotic cytokine transforming growth factor β1 (TGFβ1) and to lipotoxic palmitic acid. Together, these results suggested a role for lumican in hepatic fibrosis. To investigate the functional role of lumican in hepatic fibrosis, lumican null (Null) and wild-type (WT) littermates were administered carbon tetrachloride intra-peritoneally. Serum and liver tissue were analyzed for indices of liver injury, fibrosis, matrix turnover, and proliferation. Hepatic fibrosis was greatly reduced in null animals (P<0.05). Paradoxically, gene expression of fibrosis-related genes such as TGFβ1 and collagen 1 was numerically higher in null animals though statistically insignificant from WT animals. On the other hand, α smooth muscle actin expression (α-SMA), a marker for activated fibroblasts, the main contributors of collagen production was significantly higher (P<0.05) in null animals as compared with WT littermates. Among the matrix metalloproteases (MMP), MMP13 was significantly increased (P<0.05) in null animals. Ultra-structural imaging indicated differences in the organization and spatial distribution of hepatic collagen fibrils of null and WT mice. Cell proliferation was significantly increased (P<0.05) in null animals. We conclude that lumican is a prerequisite for hepatic fibrosis. The protective effect of lumican deficiency in hepatic fibrosis appears to be downstream of collagen production and mediated through the combined effects of impaired collagen fibrillogenesis, increased matrix turnover, and an enhanced proliferative response.
Figures







References
-
- Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III–XIII. 1–151. - PubMed
-
- Reeves HL, Friedman SL. Activation of hepatic stellate cells–a key issue in liver fibrosis. Front Biosci. 2002;7:d808–d826. - PubMed
-
- Chakravarti S, Stallings RL, SundarRaj N, et al. Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22. Genomics. 1995;27:481–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

