Disruption of wave-associated Rac GTPase-activating protein (Wrp) leads to abnormal adult neural progenitor migration associated with hydrocephalus
- PMID: 23007397
- PMCID: PMC3493966
- DOI: 10.1074/jbc.M112.398834
Disruption of wave-associated Rac GTPase-activating protein (Wrp) leads to abnormal adult neural progenitor migration associated with hydrocephalus
Abstract
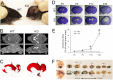
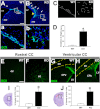
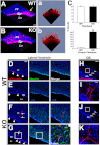
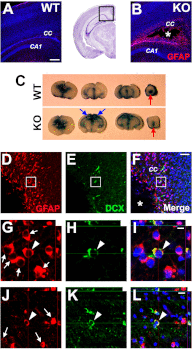
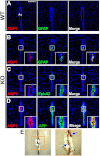
Hydrocephalus is the most common developmental disability and leading cause of brain surgery for children. Current treatments are limited to surgical intervention, as the factors that contribute to the initiation of hydrocephalus are poorly understood. Here, we describe the development of obstructive hydrocephalus in mice that are null for Wrp (Srgap3). Wrp is highly expressed in the ventricular stem cell niche, and it is a gene required for cytoskeletal organization and is associated with syndromic and psychiatric disorders in humans. During the postnatal period of progenitor cell expansion and ventricular wall remodeling, loss of Wrp results in the abnormal migration of lineage-tagged cells from the ventricular region into the corpus callosum. Within this region, mutant progenitors appear to give rise to abnormal astroglial cells and induce periventricular lesions and hemorrhage that leads to cerebral aqueductal occlusion. These results indicate that periventricular abnormalities arising from abnormal migration from the ventricular niche can be an initiating cause of noncommunicating hydrocephalus.
Figures





References
-
- Beni-Adani L., Biani N., Ben-Sirah L., Constantini S. (2006) The occurrence of obstructive vs. absorptive hydrocephalus in newborns and infants. Relevance to treatment choices. Childs Nerv. Syst. 22, 1543–1563 - PubMed
-
- Banizs B., Pike M. M., Millican C. L., Ferguson W. B., Komlosi P., Sheetz J., Bell P. D., Schwiebert E. M., Yoder B. K. (2005) Dysfunctional cilia lead to altered ependyma and choroid plexus function and result in the formation of hydrocephalus. Development 132, 5329–5339 - PubMed
-
- Davy B. E., Robinson M. L. (2003) Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum. Mol. Genet. 12, 1163–1170 - PubMed
-
- Ibañez-Tallon I., Pagenstecher A., Fliegauf M., Olbrich H., Kispert A., Ketelsen U. P., North A., Heintz N., Omran H. (2004) Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

