Pharmacokinetics of tacrolimus during pregnancy
- PMID: 23007747
- PMCID: PMC3498613
- DOI: 10.1097/FTD.0b013e3182708edf
Pharmacokinetics of tacrolimus during pregnancy
Abstract
Background: Information on the pharmacokinetics of tacrolimus during pregnancy is limited to case reports despite the increasing number of pregnant women being prescribed tacrolimus for immunosuppression.
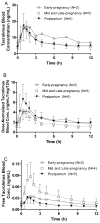
Methods: Blood, plasma, and urine samples were collected over 1 steady-state dosing interval from women treated with oral tacrolimus during early to late pregnancy (n = 10) and postpartum (n = 5). Total and unbound tacrolimus as well as metabolite concentrations in blood and plasma were assayed by a validated liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) method. A mixed-effect linear model was used for comparison across gestational age and using postpartum as the reference group.
Results: The mean oral clearance (CL/F) based on whole-blood tacrolimus concentration was 39% higher during mid-pregnancy and late pregnancy compared with postpartum (47.4 ± 12.6 vs. 34.2 ± 14.8 L/h, P < 0.03). Tacrolimus-free fraction increased by 91% in plasma (f(P)) and by 100% in blood (f(B)) during pregnancy (P = 0.0007 and 0.002, respectively). Increased fP was inversely associated with serum albumin concentration (r = -0.7, P = 0.003), which decreased by 27% during pregnancy. Pregnancy-related changes in f(P) and f(B) contributed significantly to the observed gestational increase in tacrolimus whole-blood CL/F (r² = 0.36 and 0.47, respectively, P < 0.01). In addition, tacrolimus whole-blood CL/F was inversely correlated with both hematocrit and red blood cell counts, suggesting that binding of tacrolimus to erythrocytes restricts its availability for metabolism. Treating physicians increased tacrolimus dosages in study participants during pregnancy by an average of 45% to maintain tacrolimus whole-blood trough concentrations in the therapeutic range. This led to striking increases in unbound tacrolimus trough concentrations and unbound area under the concentration-time curve, by 112% and 173%, respectively, during pregnancy (P = 0.02 and 0.03, respectively).
Conclusions: Tacrolimus pharmacokinetics are altered during pregnancy. Dose adjustment to maintain whole-blood tacrolimus concentration in the usual therapeutic range during pregnancy increases circulating free drug concentrations, which may impact clinical outcomes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
-
- Scott LJ, McKeage K, Keam SJ, et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63(12):1247–97. - PubMed
-
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs--effects on mother andchild. N Engl J Med. 2006;354(12):1281–93. - PubMed
-
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2010:65–85. - PubMed
-
- Jusko WJ, Thomson AW, Fung J, et al. Consensus document: therapeutic monitoring of tacrolimus (FK-506) Ther Drug Monit. 1995;17(6):606–14. - PubMed
-
- Machida M, Takahara S, Ishibashi M, et al. Effect of temperature and hematocrit on plasma concentration of FK 506. Transplant Proc. 1991;23(6):2753–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

