Prioritizing chemicals and data requirements for screening-level exposure and risk assessment
- PMID: 23008278
- PMCID: PMC3556628
- DOI: 10.1289/ehp.1205355
Prioritizing chemicals and data requirements for screening-level exposure and risk assessment
Abstract
Background: Scientists and regulatory agencies strive to identify chemicals that may cause harmful effects to humans and the environment; however, prioritization is challenging because of the large number of chemicals requiring evaluation and limited data and resources.
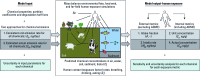
Objectives: We aimed to prioritize chemicals for exposure and exposure potential and obtain a quantitative perspective on research needs to better address uncertainty in screening assessments.
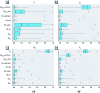
Methods: We used a multimedia mass balance model to prioritize > 12,000 organic chemicals using four far-field human exposure metrics. The propagation of variance (uncertainty) in key chemical information used as model input for calculating exposure metrics was quantified.
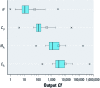
Results: Modeled human concentrations and intake rates span approximately 17 and 15 orders of magnitude, respectively. Estimates of exposure potential using human concentrations and a unit emission rate span approximately 13 orders of magnitude, and intake fractions span 7 orders of magnitude. The actual chemical emission rate contributes the greatest variance (uncertainty) in exposure estimates. The human biotransformation half-life is the second greatest source of uncertainty in estimated concentrations. In general, biotransformation and biodegradation half-lives are greater sources of uncertainty in modeled exposure and exposure potential than chemical partition coefficients.
Conclusions: Mechanistic exposure modeling is suitable for screening and prioritizing large numbers of chemicals. By including uncertainty analysis and uncertainty in chemical information in the exposure estimates, these methods can help identify and address the important sources of uncertainty in human exposure and risk assessment in a systematic manner.
Conflict of interest statement
The authors have consulted for government agencies such as Health Canada, Environment Canada, the U.K. Environment Agency, the U.S. Environmental Protection Agency, the Climate and Pollution Agency of Norway, and various chemical industry companies and organizations including ExxonMobil Biomedical Sciences, Dow Chemical, Unilever, and the European Oleochemicals and Allied Products Group. J.A.A. is currently employed by ARC Arnot Research & Consulting, a company that conducts scientific research and applied research to evaluate chemicals for their potential harmful effects to humans and the environment. J.A.A. has provided consultancy services to government agencies and the chemical industry and has received funding from government agencies and industry organizations on chemical exposure and risk assessment issues. K.B. is employed by the Norwegian Institute for Air Research, an independent, nonprofit institution offering integrated services and products within the analytical, monitoring, and consulting sectors. The authors certify that their freedom to design, conduct, interpret, and publish this analysis was not compromised by any of the sponsors of the included research.
Figures

References
-
- Arnot JA, Gobas FAPC. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in fish. Environ Rev. 2006;14:257–297.
-
- Arnot JA, Mackay D. Policies for chemical hazard and risk priority setting: can persistence, bioaccumulation, toxicity and quantity information be combined? Environ Sci Technol. 2008;42:4648–4654. - PubMed
-
- Arnot JA, Mackay D, Parkerton TF, Zaleski R, Warren CS. Multimedia modeling of human exposure to chemical substances: the roles of biomagnification and biotransformation. Environ Toxicol Chem. 2010;29:45–55. - PubMed
-
- Bennett DH, Margni MD, McKone TE, Jolliet O. Intake fraction for multimedia pollutants: a tool for life cycle analysis and comparative risk assessment. Risk Anal. 2002;22:905–918. - PubMed
-
- Cohen Hubal EA. Biologically relevant exposure science for 21st century toxicity testing. Toxicol Sci. 2009;111:226–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

