Estrogen plus progestin and colorectal cancer incidence and mortality
- PMID: 23008295
- PMCID: PMC3488271
- DOI: 10.1200/JCO.2012.42.7732
Estrogen plus progestin and colorectal cancer incidence and mortality
Erratum in
- J Clin Oncol. 2013 Jun 1;31(16):2063
Abstract
Purpose: During the intervention phase in the Women's Health Initiative (WHI) clinical trial, use of estrogen plus progestin reduced the colorectal cancer diagnosis rate, but the cancers were found at a substantially higher stage. To assess the clinical relevance of the findings, analyses of the influence of combined hormone therapy on colorectal cancer incidence and colorectal cancer mortality were conducted after extended follow-up.
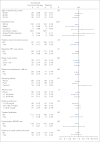
Patients and methods: The WHI study was a randomized, double-blind, placebo-controlled clinical trial involving 16,608 postmenopausal women with an intact uterus who were randomly assigned to daily 0.625 mg conjugated equine estrogen plus 2.5 mg medroxyprogesterone acetate (n = 8,506) or matching placebo (n = 8,102). Colorectal cancer diagnosis rates and colorectal cancer mortality were assessed.
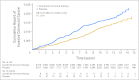
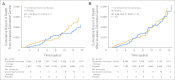
Results: After a mean of 5.6 years (standard deviation [SD], 1.03 years) of intervention and 11.6 years (SD, 3.1 years) of total follow-up, fewer colorectal cancers were diagnosed in the combined hormone therapy group compared with the placebo group (diagnoses/year, 0.12% v 0.16%; hazard ratio [HR], 0.72; 95% CI, 0.56 to 0.94; P = .014). Bowel screening examinations were comparable between groups throughout. Cancers in the combined hormone therapy group more commonly had positive lymph nodes (50.5% v 28.6%; P < .001) and were at higher stage (regional or distant, 68.8% v 51.4%; P = .003). Although not statistically significant, there was a higher number of colorectal cancer deaths in the combined hormone therapy group (37 v 27 deaths; 0.04% v 0.03%; HR, 1.29; 95% CI, 0.78 to 2.11; P = .320).
Conclusion: The findings, suggestive of diagnostic delay, do not support a clinically meaningful benefit for combined hormone therapy on colorectal cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA . 2002;288:321–333. - PubMed
-
- Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med . 2004;350:991–1004. - PubMed
-
- Lin KJ, Cheung WY, Lai JY, et al. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer . 2012;130:419–430. - PubMed
-
- Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol . 2005;23:378–391. - PubMed
-
- Solimando R, Bazzoli F, Ricciardiello L. Chemoprevention of colorectal cancer: A role for ursodeoxycholic acid, folate and hormone replacement treatment? Best Pract Res Clin Gastroenterol . 2011;25:555–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

