Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics
- PMID: 23008465
- PMCID: PMC3457622
- DOI: 10.1542/peds.2012-0498
Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics
Abstract
Objective: To determine the safety and pharmacokinetics of erythropoietin (Epo) given in conjunction with hypothermia for hypoxic-ischemic encephalopathy (HIE). We hypothesized that high dose Epo would produce plasma concentrations that are neuroprotective in animal studies (ie, maximum concentration = 6000-10000 U/L; area under the curve = 117000-140000 U*h/L).
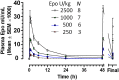
Methods: In this multicenter, open-label, dose-escalation, phase I study, we enrolled 24 newborns undergoing hypothermia for HIE. All patients had decreased consciousness and acidosis (pH < 7.00 or base deficit ≥ 12), 10-minute Apgar score ≤ 5, or ongoing resuscitation at 10 minutes. Patients received 1 of 4 Epo doses intravenously: 250 (N = 3), 500 (N = 6), 1000 (N = 7), or 2500 U/kg per dose (N = 8). We gave up to 6 doses every 48 hours starting at <24 hours of age and performed pharmacokinetic and safety analyses.
Results: Patients received mean 4.8 ± 1.2 Epo doses. Although Epo followed nonlinear pharmacokinetics, excessive accumulation did not occur during multiple dosing. At 500, 1000, and 2500 U/kg Epo, half-life was 7.2, 15.0, and 18.7 hours; maximum concentration was 7046, 13780, and 33316 U/L, and total Epo exposure (area under the curve) was 50306, 131054, and 328002 U*h/L, respectively. Drug clearance at a given dose was slower than reported in uncooled preterm infants. No deaths or serious adverse effects were seen.
Conclusions: Epo 1000 U/kg per dose intravenously given in conjunction with hypothermia is well tolerated and produces plasma concentrations that are neuroprotective in animals. A large efficacy trial is needed to determine whether Epo add-on therapy further improves outcome in infants undergoing hypothermia for HIE.
Trial registration: ClinicalTrials.gov NCT00719407.
Figures
References
-
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–338 - PubMed
-
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199(6):587–595 - PubMed
-
- Black RE, Cousens S, Johnson HL, et al. Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987 - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584 - PubMed
-
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

