Bevacizumab and Breast Cancer: A Meta-Analysis of First-Line Phase III Studies and a Critical Reappraisal of Available Evidence
- PMID: 23008712
- PMCID: PMC3447373
- DOI: 10.1155/2012/417673
Bevacizumab and Breast Cancer: A Meta-Analysis of First-Line Phase III Studies and a Critical Reappraisal of Available Evidence
Abstract
Background. Randomized studies have shown different magnitude of bevacizumab benefit in the treatment of advanced breast cancer. Regulatory agencies have modified bevacizumab treatment indications across different regions. In this study, we perform a meta-analysis of phase III studies aiming to interrogate the magnitude of bevacizumab benefit for the treatment of first-line HER2-negative metastatic breast cancer (MBC). Methods. Data from studies E2100, AVADO and RIBBON-1 were used to calculate the benefit of bevacizumab in terms of tumor overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and toxicities. Combined statistical estimates of hazard ratios (HR) and odds ratios were calculated using fixed-effects or random-effects models. Results. A total of 2,695 patients were evaluated. Combining bevacizumab with different chemotherapy backbones resulted in a 30% risk reduction of PFS events (HR = 0.70; 95% confidence interval [CI], 0.57-0.86) and increased ORR (odds ratio 1.81; 95% CI, 1.53-2.14). No OS benefit could be demonstrated (HR = 0.95; 95% CI, 0.85-1.06). Bevacizumab significantly increased the incidence of adverse events such as proteinuria, hypertension and cardiovascular events. Conclusions. Bevacizumab combined with chemotherapy in the first-line treatment of MBC significantly improved ORR and PFS, but also increased grade 3-4 toxicities. No significant OS advantage was observed.
Figures


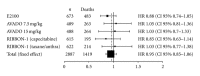
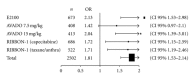
References
-
- Banerjee S, Dowsett M, Ashworth A, Martin L-A. Mechanisms of Disease: angiogenesis and the management of breast cancer. Nature Clinical Practice Oncology. 2007;4(9):536–550. - PubMed
-
- Aghajanian C, Finkler NJ, Rutherford T, et al. OCEANS: a randomized, double-blinded, placebo-controlled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC) Journal of Clinical Oncology. 2011;29(supplement 18) Abstract no. LBA5007. - PMC - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England Journal of Medicine. 2006;355(24):2542–2550. - PubMed
-
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. The Lancet. 2007;370(9605):2103–2111. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England Journal of Medicine. 2004;350(23):2335–2342. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

