Fisetin Inhibits Osteoclast Differentiation via Downregulation of p38 and c-Fos-NFATc1 Signaling Pathways
- PMID: 23008743
- PMCID: PMC3447376
- DOI: 10.1155/2012/810563
Fisetin Inhibits Osteoclast Differentiation via Downregulation of p38 and c-Fos-NFATc1 Signaling Pathways
Abstract
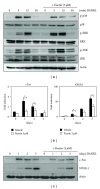
The prevention or therapeutic treatment of loss of bone mass is an important means of improving the quality of life for patients with disorders related to osteoclast-mediated bone loss. Fisetin, a flavonoid dietary ingredient found in the smoke tree (Continus coggygria), exhibits various biological activities, but its effect on osteoclast differentiation is unknown. In this study, fisetin dose-dependently inhibited the RANKL-induced osteoclast differentiation with downregulation of the activity or expression of p38, c-Fos, and NFATc1 signaling molecules. The p38/c-Fos/NFATc1-regulated expression of genes required for cell fusion and bone resorption, such as DC-STAMP and cathepsin K, was also inhibited by fisetin. Considering the rescue of fisetin's inhibitory action by NFATc1 over-expression, the cascade of p38-c-Fos-NFATc1 could be strongly involved in the inhibitory effect of fisetin on osteoclast differentiation. Furthermore, fisetin inhibited the bone-resorbing activity of mature osteoclasts. In conclusion, fisetin may be of use in the treatment of osteoclast-related disorders, including osteoporosis.
Figures



Similar articles
-
Parthenolide inhibits osteoclast differentiation and bone resorbing activity by down-regulation of NFATc1 induction and c-Fos stability, during RANKL-mediated osteoclastogenesis.BMB Rep. 2014 Aug;47(8):451-6. doi: 10.5483/bmbrep.2014.47.8.206. BMB Rep. 2014. PMID: 24314143 Free PMC article.
-
Water extract of the fruits of Alpinia oxyphylla inhibits osteoclast differentiation and bone loss.BMC Complement Altern Med. 2014 Sep 23;14:352. doi: 10.1186/1472-6882-14-352. BMC Complement Altern Med. 2014. PMID: 25249312 Free PMC article.
-
Protocatechuic Acid Attenuates Osteoclastogenesis by Downregulating JNK/c-Fos/NFATc1 Signaling and Prevents Inflammatory Bone Loss in Mice.Phytother Res. 2016 Apr;30(4):604-12. doi: 10.1002/ptr.5565. Epub 2016 Jan 20. Phytother Res. 2016. PMID: 26792397
-
The polyphenol fisetin protects bone by repressing NF-κB and MKP-1-dependent signaling pathways in osteoclasts.PLoS One. 2013 Jul 4;8(7):e68388. doi: 10.1371/journal.pone.0068388. Print 2013. PLoS One. 2013. PMID: 23861901 Free PMC article.
-
Regulators of osteoclast differentiation and cell-cell fusion.Keio J Med. 2011;60(4):101-5. doi: 10.2302/kjm.60.101. Keio J Med. 2011. PMID: 22200633 Review.
Cited by
-
Investigation of the mechanism of baicalein in the treatment of periodontitis based on network pharmacology, molecular docking and experimental validation.BMC Oral Health. 2024 Aug 23;24(1):987. doi: 10.1186/s12903-024-04740-6. BMC Oral Health. 2024. PMID: 39180042 Free PMC article.
-
Kaempferide Prevents Titanium Particle Induced Osteolysis by Suppressing JNK Activation during Osteoclast Formation.Sci Rep. 2017 Nov 30;7(1):16665. doi: 10.1038/s41598-017-16853-w. Sci Rep. 2017. PMID: 29192233 Free PMC article.
-
Oat Seedlings Extract Inhibits RANKL-Induced c-Fos/NFATc1 Transcription Factors in the Early Stage of Osteoclast Differentiation.Evid Based Complement Alternat Med. 2022 Sep 23;2022:5372459. doi: 10.1155/2022/5372459. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36193131 Free PMC article.
-
Study on the Mechanism of Treating Femoral Head Necrosis with Drynariae Rhizoma Based on Network Pharmacology.Comput Math Methods Med. 2022 Jun 6;2022:3631722. doi: 10.1155/2022/3631722. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Oct 18;2023:9785027. doi: 10.1155/2023/9785027. PMID: 35707043 Free PMC article. Retracted.
-
Alternative NF-κB Regulates RANKL-Induced Osteoclast Differentiation and Mitochondrial Biogenesis via Independent Mechanisms.J Bone Miner Res. 2015 Dec;30(12):2287-99. doi: 10.1002/jbmr.2584. Epub 2015 Aug 6. J Bone Miner Res. 2015. PMID: 26094846 Free PMC article.
References
-
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. The Journal of the American Medical Association. 2001;285(6):785–795. - PubMed
-
- Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochemical and Biophysical Research Communications. 2003;305(2):211–214. - PubMed
-
- Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochemical and Biophysical Research Communications. 2006;351(1):99–105. - PubMed
-
- Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. Journal of Bone and Mineral Research. 2002;17(10):1904–1912. - PubMed
-
- Rassi CM, Lieberherr M, Chaumaz G, Pointillart A, Cournot G. Down-regulation of osteoclast differentiation by daidzein via caspase 3. Journal of Bone and Mineral Research. 2002;17(4):630–638. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

