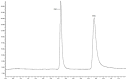
Simultaneous Estimation of Amlodipine Besylate and Indapamide in a Pharmaceutical Formulation by a High Performance Liquid Chromatographic (RP-HPLC) Method
- PMID: 23008807
- PMCID: PMC3447622
- DOI: 10.3797/scipharm.1203-07
Simultaneous Estimation of Amlodipine Besylate and Indapamide in a Pharmaceutical Formulation by a High Performance Liquid Chromatographic (RP-HPLC) Method
Abstract
An isocratic reversed-phase liquid chromatograpic assay method was developed for the quantitative determination of amlodipine besylate (AML) and indapamide (IND) in combined dosage form. A Brownlee C-18, 5 μm column with a mobile phase containing 0.02 M potassium dihydrogen phosphate-methanol (30+70, v/v) total pH-adjusted to 3 using o-phosphoric acid was used. The flow rate was 1.0 mL min(-1) and effluents were monitored at 242 nm. The retention times of amlodipine besylate and indapamide were 5.9 min and 3.6 min, respectively. The proposed method was validated with respect to linearity, accuracy, precision, and robustness. The method was successfully applied to the estimation of amlodipine besylate and indapamide in combined tablet dosage forms.
Keywords: Amlodipine; Indapamide; RP-HPLC; Tablet; Validation.
Figures
Similar articles
-
Stability Indicating RP-HPLC Estimation of Atorvastatin Calcium and Amlodipine Besylate in Pharmaceutical Formulations.Indian J Pharm Sci. 2008 Nov;70(6):754-60. doi: 10.4103/0250-474X.49117. Indian J Pharm Sci. 2008. PMID: 21369436 Free PMC article.
-
A Validated HPLC Method for Simultaneous Determination of Perindopril Arginine, Amlodipine, and Indapamide: Application in Bulk and in Different Pharmaceutical Dosage Forms.J AOAC Int. 2017 Jul 1;100(4):992-999. doi: 10.5740/jaoacint.16-0279. Epub 2017 Feb 7. J AOAC Int. 2017. PMID: 28168948
-
Simultaneous estimation of amlodipine besylate and nebivolol hydrochloride in tablet dosage forms by reverse phase-high-performance liquid chromatographic using ultraviolet detection.Pharm Methods. 2011 Jan;2(1):9-14. doi: 10.4103/2229-4708.81083. Pharm Methods. 2011. PMID: 23781423 Free PMC article.
-
Stability indicating LC-method for estimation of paracetamol and lornoxicam in combined dosage form.Sci Pharm. 2011 Jan-Mar;79(1):113-22. doi: 10.3797/scipharm.1012-03. Epub 2011 Jan 20. Sci Pharm. 2011. PMID: 21617776 Free PMC article.
-
Stability-indicating liquid chromatographic method for the quantification of the new antipsychotic agent asenapine in bulk and in pharmaceutical formulation.Sci Pharm. 2012 Apr-Jun;80(2):407-17. doi: 10.3797/scipharm.1112-07. Epub 2012 Apr 1. Sci Pharm. 2012. PMID: 22896826 Free PMC article.
Cited by
-
Development of UV spectrophotometry methods for concurrent quantification of amlodipine and celecoxib by manipulation of ratio spectra in pure and pharmaceutical formulation.PLoS One. 2019 Sep 16;14(9):e0222526. doi: 10.1371/journal.pone.0222526. eCollection 2019. PLoS One. 2019. PMID: 31525229 Free PMC article.
-
Method development and validation of HPLC tandem/mass spectrometry for quantification of perindopril arginine and amlodipine besylate combination in bulk and pharmaceutical formulations.Res Pharm Sci. 2017 Aug;12(4):307-314. doi: 10.4103/1735-5362.212048. Res Pharm Sci. 2017. PMID: 28855942 Free PMC article.
-
Development and Validation of highly Sensitive Stability Indicating Spectrofluorimetric Method for Determination of Amlodipine in Pharmaceutical Preparations and Human Plasma.J Fluoresc. 2016 Nov;26(6):2141-2149. doi: 10.1007/s10895-016-1910-4. Epub 2016 Aug 26. J Fluoresc. 2016. PMID: 27566613
References
-
- O’Neil MJ. The Merck Index. 14th Ed. Merck & Co. Inc.; Whitehouse Station, NJ: 2006.
-
- Anthony CM, Osselton MD. Clarke’s analysis of Drugs and poisons in body fluids and post-mortem material. 3rd edition. Vol. 2. Pharmaceutical press; 2004. p. 629.p. 1132.
-
- Indian Pharmacopoeia 2007-Govt. of India, The Indian pharmacopoeia commission. Ghaziabad: Ministry of Health & Family welfare;
-
- Sharma BK. Instrumental Methods of Chemical Analysis. 20th edition. GOEL Publishing House; 2005.
-
- Q2 (R1), Text on Validation of Analytical Procedures. International Conference on Harmonization (ICH); Geneva, Switzerland. 2005.
LinkOut - more resources
Full Text Sources
Miscellaneous