Stereoselective C-glycoside formation with 2-O-benzyl-4,6-O-benzylidene protected 3-deoxy gluco- and mannopyranoside donors: comparison with O-glycoside formation
- PMID: 23009024
- PMCID: PMC3501215
- DOI: 10.1021/jo3011655
Stereoselective C-glycoside formation with 2-O-benzyl-4,6-O-benzylidene protected 3-deoxy gluco- and mannopyranoside donors: comparison with O-glycoside formation
Abstract
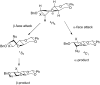
Unlike alcohols, the reaction of C-nucleophiles with 2-O-benzyl-4,6-O-benzylidene-protected 3-deoxy-gluco- and mannopyranosyl thioglycosides is highly stereoselective providing the α-C-glycosides in the gluco-series and the β-C-glycosides in the manno-series. Conformational analysis of nucleophilic attack of putative intermediate glycosyl oxocarbenium ions suggests that the observed selectivities for C-glycoside formation can be explained by preferential attack on the opposite face of the oxocarbenium to the C2-H2 bond and that eclipsing interactions with this bond are the main stereodetermining factor. It is argued that the steric interactions in the attack of alcohols (sp(3)-hybridized O) and of typical carbon-based nucleophiles (sp(2) C) on oxocarbenium ions are very different, with the former being less severe, and thus that there is no a priori reason to expect O- and C-glycosylation to exhibit parallel stereoselectivities for attack on a given oxocarbenium ion.
Figures






References
-
- Levy DE, Tang C. The Chemistry of C-Glycosides. Pergamon; Oxford: 1995.
- Postema MHD, editor. C-Glycoside Synthesis. CRC; Boca Raton: 1995.
- Postema MHD, Calimente D. In: Glycochemistry: Principles, Synthesis, and Applications. Wang PG, Bertozzi CR, editors. Dekker; New York: 2001. pp. 77–131.
-
- Nukada T, Bérces A, Wang L, Zgierski MZ, Whitfield DM. Carbohydr Res. 2005;340:841–852. - PubMed
- Ionescu AR, Whitfield DM, Zgierski MZ, Nukada T. Carbohydr Res. 2006;341:2912–2920. - PubMed
- Whitfield DM, Nukada T. Carbohydr Res. 2007;342:1291–1304. - PubMed
- Whitfield DM. Adv Carbohydr Chem Biochem. 2009;62:83–159. - PubMed
-
- Crich D, Cai W, Dai Z. J Org Chem. 2000;65:1291–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

