Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth
- PMID: 23009203
- PMCID: PMC3562128
- DOI: 10.1021/jm301098e
Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth
Abstract
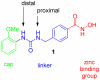
The incidence of malignant melanoma has dramatically increased in recent years thus requiring the need for improved therapeutic strategies. In our efforts to design selective histone deactylase inhibitors (HDACI), we discovered that the aryl urea 1 is a modestly potent yet nonselective inhibitor. Structure-activity relationship studies revealed that adding substituents to the nitrogen atom of the urea so as to generate compounds bearing a branched linker group results in increased potency and selectivity for HDAC6. Compound 5 g shows low nanomolar inhibitory potency against HDAC6 and a selectivity of ∼600-fold relative to the inhibition of HDAC1. These HDACIs were evaluated for their ability to inhibit the growth of B16 melanoma cells with the most potent and selective HDAC6I being found to decrease tumor cell growth. To the best of our knowledge, this work constitutes the first report of HDAC6-selective inhibitors that possess antiproliferative effects against melanoma cells.
Figures


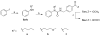
Similar articles
-
Structure-activity relationship and mechanistic studies for a series of cinnamyl hydroxamate histone deacetylase inhibitors.Bioorg Med Chem. 2021 Apr 1;35:116085. doi: 10.1016/j.bmc.2021.116085. Epub 2021 Feb 23. Bioorg Med Chem. 2021. PMID: 33668008
-
Investigation of the effect of different linker chemotypes on the inhibition of histone deacetylases (HDACs).Bioorg Chem. 2021 Jan;106:104462. doi: 10.1016/j.bioorg.2020.104462. Epub 2020 Nov 6. Bioorg Chem. 2021. PMID: 33213894
-
Effect of phenylurea hydroxamic acids on histone deacetylase and VEGFR-2.Bioorg Med Chem. 2021 Nov 15;50:116454. doi: 10.1016/j.bmc.2021.116454. Epub 2021 Oct 4. Bioorg Med Chem. 2021. PMID: 34634618
-
Beyond the Selective Inhibition of Histone Deacetylase 6.Mini Rev Med Chem. 2016;16(14):1175-84. doi: 10.2174/1389557516666160428115959. Mini Rev Med Chem. 2016. PMID: 27121714 Review.
-
Histone deacetylase as a new target for cancer chemotherapy.Cancer Chemother Pharmacol. 2001 Aug;48 Suppl 1:S20-6. doi: 10.1007/s002800100300. Cancer Chemother Pharmacol. 2001. PMID: 11587361 Review.
Cited by
-
HDACs and HDAC Inhibitors in Cancer Development and Therapy.Cold Spring Harb Perspect Med. 2016 Oct 3;6(10):a026831. doi: 10.1101/cshperspect.a026831. Cold Spring Harb Perspect Med. 2016. PMID: 27599530 Free PMC article. Review.
-
Histone deacetylase 6 plays an important role in TGF-β-induced murine Treg cell differentiation by regulating cell proliferation.Sci Rep. 2022 Dec 29;12(1):22550. doi: 10.1038/s41598-022-27230-7. Sci Rep. 2022. PMID: 36581745 Free PMC article.
-
Improving TRAIL-induced apoptosis in cancers by interfering with histone modifications.Cancer Drug Resist. 2020 Oct 9;3(4):791-803. doi: 10.20517/cdr.2020.58. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582230 Free PMC article. Review.
-
Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry.J Med Chem. 2020 Mar 26;63(6):2751-2788. doi: 10.1021/acs.jmedchem.9b01541. Epub 2019 Dec 2. J Med Chem. 2020. PMID: 31789518 Free PMC article.
-
Structure-based design generated novel hydroxamic acid based preferential HDAC6 lead inhibitor with on-target cytotoxic activity against primary choroid plexus carcinoma.J Enzyme Inhib Med Chem. 2019 Dec;34(1):1062-1077. doi: 10.1080/14756366.2019.1613987. J Enzyme Inhib Med Chem. 2019. PMID: 31072216 Free PMC article.
References
-
- Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Molecular and Cellular Biology. 2008;28(5):1688–1701. - PMC - PubMed
-
- Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, van Duzer JH, Mazitschek R, Ogier WC, Cirstea D, Rodig S, Eda H, Scullen T, Canavese M, Bradner J, Anderson KC, Jones SS, Raje N. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579–2589. - PMC - PubMed
-
- Peltonen K, Kiviharju TM, Jarvinen PM, Ra R, Laiho M. Melanoma cell lines are susceptible to histone deacetylase inhibitor TSA provoked cell cycle arrest and apoptosis. Pigment Cell Research. 2005;18(3):196–202. - PubMed
- Facchetti F, Previdi S, Ballarini M, Minucci S, Perego P, La Porta CAM. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis. 2004;9(5):573–582. - PubMed
-
- Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. Journal of Biological Chemistry. 2002;277(28):25748–25755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

