Biological rationale for the design of polymeric anti-cancer nanomedicines
- PMID: 23009337
- PMCID: PMC4605218
- DOI: 10.3109/1061186X.2012.723213
Biological rationale for the design of polymeric anti-cancer nanomedicines
Abstract
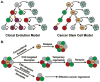
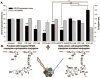
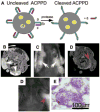
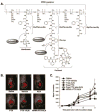
Understanding the biological features of cancer is the basis for designing efficient anti-cancer nanomedicines. On one hand, important therapeutic targets for anti-cancer nanomedicines need to be identified based on cancer biology, to address the unmet medical needs. On the other hand, the unique pathophysiological properties of cancer affect the delivery and interactions of anti-cancer nanomedicines with their therapeutic targets. This review discusses several critical cancer biological properties that challenge the currently available anti-cancer treatments, including cancer heterogeneity and cancer stem cells, the complexcity of tumor microenvironment, and the inevitable cancer metastases. In addition, the biological bases of the enhanced permeability and retention (EPR) effect and tumor-specific active targeting, as well as the physiological barriers for passive and active targeting of anti-cancer nanomedicines are covered in this review. Correspondingly, possible nanomedicine strategies to target cancer heterogeneity, cancer stem cells and metastases, to overcome the challenges related to tumor passive targeting and tumor penetration, and to improve the interactions of therapeutic payloads with the therapeutic targets are discussed. The focus is mainly on the designs of polymeric anti-cancer nanomedicines.
Figures





Similar articles
-
Can nanomedicines kill cancer stem cells?Adv Drug Deliv Rev. 2013 Nov;65(13-14):1763-83. doi: 10.1016/j.addr.2013.09.016. Epub 2013 Oct 10. Adv Drug Deliv Rev. 2013. PMID: 24120657 Free PMC article. Review.
-
[The development of novel tumor targeting delivery strategy].Yao Xue Xue Bao. 2016 Feb;51(2):272-80. Yao Xue Xue Bao. 2016. PMID: 29856581 Review. Chinese.
-
To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?J Control Release. 2016 Dec 28;244(Pt A):108-121. doi: 10.1016/j.jconrel.2016.11.015. Epub 2016 Nov 18. J Control Release. 2016. PMID: 27871992 Review.
-
Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment.Acc Chem Res. 2019 Jul 16;52(7):1771-1782. doi: 10.1021/acs.accounts.9b00136. Epub 2019 Jun 26. Acc Chem Res. 2019. PMID: 31241894
-
DePEGylation strategies to increase cancer nanomedicine efficacy.Nanoscale Horiz. 2019 Mar 1;4(2):378-387. doi: 10.1039/c8nh00417j. Epub 2018 Dec 11. Nanoscale Horiz. 2019. PMID: 32254090 Review.
Cited by
-
Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer.Pharmaceutics. 2020 Jul 3;12(7):621. doi: 10.3390/pharmaceutics12070621. Pharmaceutics. 2020. PMID: 32635231 Free PMC article.
-
Polymer nanomedicines.Adv Drug Deliv Rev. 2020;156:40-64. doi: 10.1016/j.addr.2020.07.020. Epub 2020 Jul 28. Adv Drug Deliv Rev. 2020. PMID: 32735811 Free PMC article. Review.
-
Design of smart HPMA copolymer-based nanomedicines.J Control Release. 2016 Oct 28;240:9-23. doi: 10.1016/j.jconrel.2015.10.003. Epub 2015 Oct 3. J Control Release. 2016. PMID: 26437260 Free PMC article. Review.
-
Hydrophilic biomaterials: From crosslinked and self-assembled hydrogels to polymer-drug conjugates and drug-free macromolecular therapeutics.J Control Release. 2024 Sep;373:1-22. doi: 10.1016/j.jconrel.2024.05.012. Epub 2024 May 17. J Control Release. 2024. PMID: 38734315
-
Polymer-drug conjugate therapeutics: advances, insights and prospects.Nat Rev Drug Discov. 2019 Apr;18(4):273-294. doi: 10.1038/s41573-018-0005-0. Nat Rev Drug Discov. 2019. PMID: 30542076 Free PMC article. Review.
References
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
